Want to experience the greatest in board studying? Check out our interactive question bank podcast- the FIRST of its kind here: emrapidbombs.supercast.com
Author: Blake Briggs, MD
Peer reviewer: Mary Claire O’Brien, MD
Objectives: the learner will understand the classifications of aortic dissection; its pathophysiology, risk factors, presentation, as well as diagnosis and acute management.
Introduction
Acute aortic syndromes are a collection of life-threatening aortic pathologies, ranging from hematoma to dissection.
This review covers aortic dissection, a deceptively difficult diagnosis that is rare but lives in our nightmares. Its rate is about 3 per 100,000 person-years.
Aortic dissection is difficult to diagnose. One autopsy study showed 63% of patients of 388 total who were not diagnosed with dissection prior to death.
Aortic dissection causes death by several mechanisms. It can be related to the rupture of the dissection into the pericardium, obstruction of the coronary ostia leading to infarction, and end-organ ischemia due to lack of oxygenated blood flow. Mortality for surgically managed Stanford A dissections is around 20%, medically managed 30-day mortality for Type A is 30%.
Classification
Accelerate your learning with our EM Question Bank Podcast
- Rapid learning
- Interactive questions and answers
- new episodes every week
- Become a valuable supporter
There are 2 main classifications of dissection, Stanford and DeBakey. Stanford is more commonly used.
Stanford Type A: Ascending Aorta (may progress to Arch). (look at all those A’s).
-Twice as common as Type B.
-30% of cases involve the arch.
Stanford Type B: descending aorta.
Pathophysiology
Tear in the aortic intima (inner layer of aortic wall), allowing blood to enter the aortic media via the tear, creating a false lumen. From here, the tear can basically travel proximally or distally much like a zipper on a jacket. Except unzipping this jacket causes death.
If the tear moves proximally it will cause the multitude of ischemic complications like aortic regurgitation, tamponade, and coronary ischemia. Moving distally, the various mesenteric and renal vessels will be affected, causing end-organ ischemia.
As this is occurring, PHYSICS(!) are at work (we capitalized that word for emphasis).
There is this phenomenon of “intrinsic true lumen collapse”, where the thin walled false lumen area increases in size and is directly proportional to the BP. It continues to expand in size and therefore the true lumen collapses due to the pressure differential. This is why BP control is so critical, it decreases false lumen expansion.
Risk factors
By far the most common risk factor is hypertension. ~75%of patients with aortic dissection have a history.
The more abrupt increase in BP, the highest the risk of dissection. Crack cocaine and methamphetamines have been found in nearly 37% of dissections from an urban population in one study.
Aortic aneurysm: thoracic aneurysms (especially ascending) associated with nearly 13-20% of patients.
Bicuspid aortic valve: higher risk of dissection likely due to enlarged aortic root.
Obvious genetic causes: Marfan’s (present in 50% of patients <40 years old), Ehlers-Danlos, Turner Syndrome. These conditions are associated with this crazy high yield board buzzword called “cystic medial necrosis”, which is pathognomonic for aortic dissection.
Presentation
Patients are typically older and in their 60s-80s.
Women, like acute coronary syndrome, often have a delayed presentation and are older than men.
Symptoms can often differ in occurrence based on the location of the dissection.
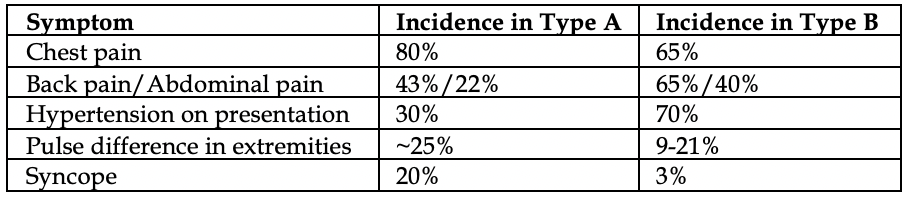
Acute pain is the most common symptom, found in 90% of patients. Sharp is the most common descriptor (68%), tearing (50%).
Hypertension is common in Type B dissections (70%), but sadly roughly 30% in Type A (ouch).
“Painless” dissections have been reported but are rare (<7% in one retrospective review alone). Diabetes is most common risk factor for a painless dissection (and you would say of course it is).
Pulse deficit between arms or legs: not reliable. Its absence by no means excludes dissection. However, its presence is concerning for increased mortality. In one study 24 hours from presentation, 9% of those without pulse deficits died, 15% of those with 1-2 deficits died, and 35% of those with 3+ deficits died.
BP deficit between upper extremities: variation by >20 mmHg is considered significant. Not commonly found and not reliable.
New murmur: reflects acute aortic regurgitation. Diastolic murmur with wide pulse pressure. Very specific for Type A, (50-66%).
Focal neurologic deficits are concerning for dissection extension into the carotid arteries. Spinal cord ischemia, Horner syndrome, and stroke-symptoms are less common but are extremely poor prognostic indicators.
Hypotension: get ready to have a bad shift. This indicates rupture, aortic valve disruption, or tamponade.
Syncope: these patients are more likely to have underlying stroke, early death in 24 hours, and tamponade.
So who is high risk? Who do we work up?
This is a rare diagnosis but catastrophic. Here’s an approach from EM Cases, Anton Helman.
Ask the patient these 3 questions: Quality of pain? (sharp > tearing), Abrupt onset?, Radiation to back/belly?
If all 3 questions are positive, dissection was suspected in 91%! If <3, only ~50%.
One needs to think of dissection as “chest pain +” or CP+. These patients won’t just have chest pain. CP+ syncope, CP+ neuro deficit, CP+ back pain, etc.
If you see a chest pain or neuro patient with “extra stuff” that does not fit their standard disease schema, have a high suspicion.
Diagnosis
EKG: no specific findings. Normal in 30% of patients. 42% showed nonspecific ST and T wave changes. Evidence of MI in ascending dissection was in 5%
CXR: everyone talks about the widened mediastinum but in reality, its absence does NOT exclude a dissection from occurring. Only ~60% of the time it is present.
Look for loss of the Aortic knob (flattening of the aortic arch). Look for Calcium sign (white line of calcium within aortic knob measured to outer edge of knob. >0.5 cm concerning for dissection).
D-dimer: research has been looking at this test as a sensitive screening method. Like PE, d-dimer might be a useful test for those who do NOT have a dissection (low suspicion and wish to rule it out). Patients who have a dissection with a negative D-dimer (0.4%). Its promising but this has NOT been validated yet, and alone should not be relied upon with a convincing clinical presentation.
CT angiography: should include chest and abdomen. Look for intimal flap separating false from true lumen. Sensitivity of 83-95% and specificity 87-100%. The lower ends of those percentiles are dependent on contrast infusion timing, in particular at the base of the ascending aorta.
TEE: 98% sensitive, 63-96% specific. Has basically been supplanted in the ED by wide availability and speed of CT. TTE can be performed in the OR.
MR angiography: most accurate test, not commonly done.
Treatment
This is the truest form of hypertensive emergency. RAPID lowering is needed as fast as possible in order to prevent false lumen expansion.
Target: “as low as possible”, but realistically 100-120 systolic in <30 minutes, HR <60.
Agent of choice: Esmolol > Labetalol > Diltiazem > Nicardipine/Clevidipine > Nitroprusside
*Clevidipine is faster onset and usually cheaper than nicardipine
Esmolol dosing: 500 mcg/kg loading dose in 1 minute. Infuse at 25-50 mcg/kg/minute; maximum of 300 mcg/kg/minute
Labetalol dosing: 20 mg loading dose, inject 20-80 mg every 10 minutes to a total dose of 300 mg
Fentanyl boluses need to be occurring simultaneously with BP control as it reduces sympathetic pain response which increases HR and BP.
Agents NOT to use: Nitroglycerin, hydralazine, or other vasodilators which cause increase in inotropy.
Specific longer-term therapies differ based on location.
Type A: surgical emergency due to the multitude of structural complications (tamponade, valve regurgitation, etc). Surgery includes excising the intimal tear, obliterating false lumen entry, placing a graft, and repairing/replacing the aortic valve (sounds like a busy case).
Type B: medical emergency. Manage BP and admit to CVICU. Vascular surgery follows and surgery is reserved for specific situations.
Prognosis:
The development of malperfusion in Type A dissections has mortality rates up to 44%. Prompt surgical therapy is required.
10-year actual survival rate after optimal treatment is 30-88%, both Types are similar.



