Want to experience the greatest in board studying? Check out our interactive question bank podcast- the FIRST of its kind here: emrapidbombs.supercast.com
Author: Blake Briggs, MD
Peer Reviewer: Mary Claire O’Brien, MD
Sudden cardiac arrest (SCA): sudden and unexpected cessation of cardiac activity where the patient becomes unresponsive and lacks a pulse. It is the most common cause of “dropping dead” in the world. It accounts for ~450,000 deaths in the US alone per year. In those >40 years old, ventricular fibrillation from an MI is the most common cause. In those <40, ventricular fibrillation secondary to hypertrophic obstructive cardiomyopathy (HOCM) is the most common cause in the US. Males are 2-3 times more likely to experience SCA than women. Risk of SCA is increased 10-fold in the presence of coronary artery disease, and 4-fold increase if patient has cardiac disease risk factors.
Fast facts: without CPR and defibrillation, survival from cardiac arrest caused by VF declines by approximately 10% for each minute, and after more than 12 minutes without CPR, the survival rate is <5%. Survival to hospital discharge for those treated between 1998 and 2001 has not significantly changed, and this can most likely be contributed to time of CPR onset at the scene. It cannot be understated how education regarding bystander CPR needs to be a priority in prehospital care.
What causes people to drop dead?
Causes of SCA: broken up into several categories for ease of organization. First, think cardiac vs noncardiac. Cardiac is by far the most common cause. Secondly, is it because of a structural (muscular) issue in the heart or electrical (nonstructural) conduction? Finally, if structural, is the cause due to ischemic heart disease or non-ischemic heart disease? Overall, IHD/CAD is the most common cause of SCA.
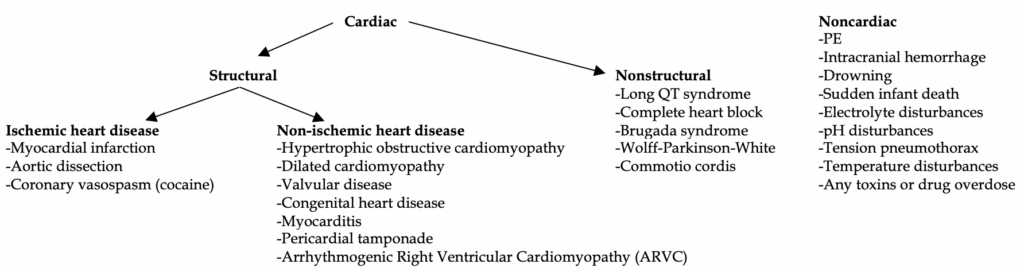
Depending on the patient’s age, some diseases are more common than others.
<35 years old: HOCM is the most common (50% of the time), followed by other major causes like congenital heart disease, nonstructural heart disease, cocaine use, and dissection– in no particular order.
Accelerate your learning with our EM Question Bank Podcast
- Rapid learning
- Interactive questions and answers
- new episodes every week
- Become a valuable supporter
>35 years old: MI (80% of the time!), followed by PE, dissection, and dilated cardiomyopathy as major causes.
This simple point underlies a major board question topic: Patients <35 years old who present on board exams with chest pain do NOT have acute coronary syndrome. Studies have shown there is a <10% of MI occur in those <35 years old, which means on board tests you will never see a patient <35 with chest pain due to MI or angina. Of course, in real life there are people in their 20’s who present with MI, so never ignore them if it’s a concerning story!
Pathophysiologic course of SCA
Loss of oxygenated blood flow to coronary arteries either due to 1) structural heart disease, 2) electrical pathology, 3) noncardiac cause à loss of consciousness in seconds-minutes due to cerebral ischemia à development of tachyarrhythmia (most commonly ventricular fibrillation) à loss of heart function with eventual death.
Generalized presentation: symptoms and warning signs are uncommon. Which is quite scary. Only 50% of the time a patient can display signs of chest pain or 20% of the time display dyspnea.
Brief review of the high yield offenders…
Myocardial infarction: due to >70% stenosis of the coronary arteries. 50% of the time a patient has an MI, they develop cardiac arrest. The other 50% the patient will be conscious and (usually) present to the ED with associated symptomatology. See our handouts on NSTEMI and STEMI.
Aortic Dissection: most common risk factor is hypertension. Patients on boards will typically have “tearing” chest pain that radiates to the back. In reality, “sharp” central chest pain is the most common presentation. The classic widened mediastinum on x-ray is not reliable and only found ~60% of the time. Patients present hypertensive 70% of the time if Type B, 30% if Type A. In those <35 years old, connective tissue disorders such as Marfan’s or Ehlers-Danlos are classic on boards. Pulse deficits are unreliable on exam, BP cuff differences in arms is unreliable and uncommon. Syncope, new neurologic deficit, and hypotension are poor prognostic indicators. If stabilized rush to CT for chest/abdomen/pelvis angiography. If unable to get to CT, bedside echo might reveal widened aortic root, tamponade, active arch dissection.
Aggressive beta blocker therapy is warranted immediately if suspected!
EKG findings: nothing specific. Normal in 30% of patients. ~40% showed nonspecific ST and T wave changes. Evidence of MI in ascending dissection was in 5%.
See our handout on aortic dissection, “On a Tear” for more details on this.
Hypertrophic Obstructive Cardiomyopathy: genetic defect in sarcomere genes (classically beta-myosin), resulting in a disordered hypertrophy of the myocardium. Eventually, the enlarged myocardial wall impairs diastolic filling, causes LV outflow obstruction, and resultant ischemia. During states of increased left ventricular contractility, the thick musculature on the septum can press against the mitral valve leaflet and prevent outflow of blood into the aorta. This results in coronary ischemia and eventual fatal tachydysrhythmia. Most patients are asymptomatic until the episodes occur during exertion. Dyspnea on exertion is the most common presenting symptom, present in 90% of patients. Chest pain and syncope are only seen in 30% and 20%, respectively, but the latter is a major risk for SCA. Echo is diagnostic.
EKG findings: <10% have a normal EKG. Great screening test. Prominent Q waves in inferior and lateral leads reflects septal depolarization of the large, hypertrophied muscle. P wave enlargement, signs of LVH and LAD, late in disease one can see giant inverted T waves in V2-4.
Listen to our podcast on HOCM on iTunes or Soundcloud “Episode 55. HOCM Horns”
Long QT Syndrome: Prolongation of the QT interval (>430 in men; >450 in women) delays repolarization in cardiac myocytes and leads to torsades de pointes, a form of polymorphic ventricular tachycardia (polymorphic because it changes in amplitude). Long QT can be acquired or inherited. Inherited conditions include the infamous Romano-Ward and Jervell-Lange-Neilson disorders, both of which have Na-K channel defects. Jervell-Lange-Nielson also causes sensorineural hearing loss.
Acquired conditions are much more common and more well known. Think electrolyte issues and drugs.
-Electrolyte issues: hypokalemia, hypocalcemia, hypomagnesemia
-certain medications: Sotalol, Risperidone (and other antipsychotics), Macrolides, Chloroquine, Protease inhibitors, Quinidine, Thiazides
Wolff-Parkinson-White: an accessory pathway (Bundle of Kent) that allows electrical conduction to bypass the AV node and stimulate the ventricles. It can trigger deadly tachydysrhythmias, either narrow regular (orthodromic), or wide irregular (antidromic) depending on the route the impulses take through the accessory pathway. The HR will classically be >150 bpm, therefore preventing the effective diastolic filling and eventual coronary ischemia and infarction.
EKG: at baseline there can be a classic Delta wave- shortened PR interval with an upstroke of the Q wave instead of its normal dip downward. It is not always evident, and a shortened PR alone should raise suspicion in the right clinical context.
See handout “Out of Breath- Approach to Management of Tachyarrhythmias”
Commotio Cordis: exceedingly rare, but classic on pediatric board questions. Physical impact to the chest wall causes the heart to enter ventricular fibrillation. Children are more prone due to their immature thoracic cage. Classically the child will be in a baseball game or some sporting event where they are hit square in the chest by a ball or puck or another player.
Pulmonary Embolus: massive emboli, in simple terms, are those which cause the patient to be hemodynamically unstable, and can often block the enter pulmonary artery, especially at the pulmonary trunk (i.e. saddle embolus).
These emboli require thrombolytics (although this a hotly debated topic in medicine).
See our handout on PE “Saddle Up: All About Pulmonary Embolism”
Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC): paroxysmal ventricular arrhythmias lead to sudden death. More common in patients of Mediterranean descent. Basically, there is fibrous-fat infiltration of the right ventricle of the myocardium. It is the 2nd most common cause of death in those <35 after HOCM. It has a similar presentation to HOCM as well: palpitations, syncope, exertional dyspnea, etc. The older patients get, the more the RV will begin to falter leading to biventricular dilated cardiomyopathy. Echocardiography should be performed but high rate of false negatives. Most accurate is cardiac MRI.
EKG: epsilon wave (only seen in ~1/3 of patients), T wave inversions (>80% of patients), prolonged S wave upstroke in V1-V3.
Tx: urgent ICD placement. Antiarrhythmic therapy until ICD is warranted in most cases (e.g. sotalol, BB’s).
Brugada Syndrome: mutation in sodium channel, leading to unexplained sudden cardiac death, especially at night.
Type 1 Brugada sign (coved STE >2mm in >1 V1-3 with a NEGATIVE T wave). Must be associated with one of these to make the diagnosis: VF or polymorphic VT, FH of SCA <50, same EKG in family members, syncope, nocturnal agonal respiration.
Type 2 Brugada: saddleback STE in V1-V3.
Type 3 is a mix of the first two with <2 mm.
Tx: urgent ICD placement. Antiarrhythmic therapy until ICD is warranted in most cases (e.g. sotalol, BB’s).



