Want to experience the greatest in board studying? Check out our interactive question bank podcast- the FIRST of its kind here: emrapidbombs.supercast.com
Authors: Blake Briggs, MD
Peer Reviewer: Mary Claire O’Brien, MD
Objectives: the learner will be able to explain the pathophysiology of PE, identify the variable presentation of PE, recognize and carry out the cost-effective diagnostic algorithm of PEs, initiate correct treatment based on hemodynamic stability.
Introduction
A PE is a common form of venous thromboembolism that obstructs the pulmonary artery and its branches. We will focus on the classic VTE form of PE, the other causes (air, septic emboli, fat, etc.) are outside the scope of this review. We can classify PE’s based on timing, location, severity of symptoms.
PE’s can be acute/subacute (~days-weeks), or chronic (years).
Their location is in the order of most common: lobar/segmental > subsegmental > saddle.
Originally, it was thought saddle emboli were most associated with causing hemodynamic instability, but recent data suggests otherwise. A saddle embolus ≠ “massive PE”.
Massive PE = any PE that causes hemodynamic instability.
Accelerate your learning with our EM Question Bank Podcast
- Rapid learning
- Interactive questions and answers
- new episodes every week
- Become a valuable supporter
Submassive PE = any PE that causes greater than mild RV strain.
Pathophysiology: PE is caused by one or more aspects of Virchow’s Triad:
1) Stasis: Any type of immobilization (long drives/flights/train trips, recent surgery).
2) Hypercoagulable state or 3) Endothelial injury: pregnancy, cancer, sepsis, fractures, contraceptive pills, or genetics. The most common inherited clotting disorder is Factor V Leiden. Prothrombin G20210A, Protein C&S deficiency, and Antithrombin III deficiency are other risk factors. Really, any general inflammatory state can be considered hypercoagulable.
The majority of PEs arise from VTE in the proximal legs. In fact, about 50% of patients with proximal DVT also have a PE on presentation.
PE’s can result in significant pathology:
-infarction: segmental or subsegmental PEs. Classically can have Hampton’s hump on chest x-ray. Hemoptysis and pleuritic chest pain common
-V/Q mismatch
-RV strain: increased pulmonary vascular resistance works against the RV and decreases cardiac output.
Presentation
Most common symptom: dyspnea > pleuritic pain > calf pain/swelling > cough …orthopnea, hemoptysis
Most common sign: tachypnea > calf swelling > tachycardia > crackles/rales > murmurs/JVD…
Pearls in the HPI: true exertional dyspnea. Instead of asking “do you feel short of breath when exerting yourself?” ask “describe what triggers your dyspnea?” or “give me an example when you feel most short of breath.”
Onset of dyspnea is usually sudden and <1 hour.
PE is also a common cause of sudden cardiac death (~8%) of patients, so suspicion should always be high in those presenting with cardiac arrest.
Syncope has been found to not be a major risk factor associated with PE. In 2 large studies, PE prevalence was only about ~1%.
Certain patient populations like those with CHF or COPD can make it difficult to diagnose PE. A recent study suggested up to ~20% of patients with COPD exacerbations had some type of PE (granted this was a single center and some PEs were only subsegmental). Best advice here is to work up their primary complaint and if there is lack of improvement or symptoms/signs suggesting PE > CHF/COPD, do not hesitate to work it up.
Diagnosis
Accessory tests that are usually ordered in dyspneic patients: CBC, BNP, CMP, blood gas, troponin, EKG, CXR.
EKG: Forget what you learned in medical school. The most common finding associated with PE is “normal sinus rhythm” and the most common abnormal finding is “sinus tachycardia” followed by ST segment and T wave changes. The most specific sign for PE on EKG is flipped T waves in anterior and inferior leads.
What about that S1Q3T3? Turns out it is not specific at all for PE, however if you see it on EKG in a dyspneic patient, PE workup is not a bad idea.
Other rare EKG findings include evidence of new right heart strain: RVH, RBBB (both <10%)
CXR: most commonly normal. Rarely, a Hampton’s Hump (hump-shaped opacity in bottom lateral lower quadrant in either lung and often confused with PNA) or Westermark Sign (sharp cutoff with pulmonary vessels; “blackout”) can be seen. These both are rare.
In general CXR is not going to help you with PE but assist in ruling out other differential diagnoses.
Before proceeding with diagnostic testing, you need to stratify your patient based on risk.
The following diagnostic algorithms are based on the patient’s hemodynamic stability (mainly BP):
Hemodynamically STABLE nonpregnant patient
The goal is to efficiently diagnose PE and limit unnecessary testing. We apply Well’s, PERC, D-dimer, and CTPA (see figure 1 below). At the end of this document, we will discuss the evidence behind these tools if you want to learn more.
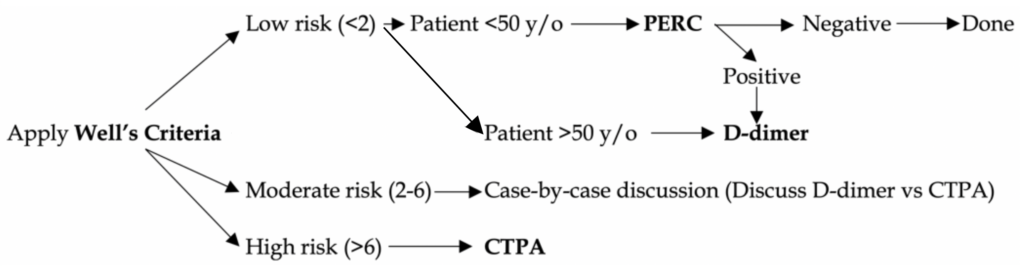
Hemodynamically UNSTABLE patient:
-High suspicion for PE immediate anticoagulation with continued supportive care until able to proceed with CTPA.
Bedside echocardiography can potentially show evidence of right heart strain. Wall abnormalities, increased RV size, abnormal septal wall motion, decreased RV function, are all not specific for PE but present in nearly 40% of PE patients. In general RV strain is both poorly sensitive and specific (53% and 61% respectively).
Treatment
Untreated PE has an associated mortality up to 30%. Anticoagulation clearly reduces mortality and morbidity. Most deaths occur in the first week from shock (massive PE group), and the other groups can have complicated, more chronic issues (e.g. pulmonary hypertension, poor gas exchange, etc).
Hemodynamically stable: initiate anticoagulation after confirmed PE diagnosis. LMW in those where oral intake is not feasible. Otherwise, rivaroxaban, apixaban, or warfarin should be discussed. Their risks, benefits, and cost are outside the scope of this handout.
Hemodynamically unstable:
Thrombolytics: Plasminogen to plasmin (accelerated thrombolysis). tPA is a natural plasma enzyme which binds to fibrin and enhances affinity for plasminogen as well as plasminogen activation. tPA has been associated with early hemodynamic improvement but obviously at cost of major bleeding.
Definite indications: persistent hypotension or shock (most widely accepted viewpoint) = “massive” PE
Few studies but overall there is improved mortality in these patients.
Considerations as case-by-case basis: severe RV dysfunction (“submassive” PE), cardiac arrest due to PE, free-floating right heart thrombus
By far, most controversial finding: right heart strain without hypotension. No randomized trials have shown mortality benefit, but there have been studies demonstrating those with RV dysfunction and a submassive PE have worse outcomes than those without RV strain. Better studies are likely coming soon and it is in our opinion this should be handled case-by-case but leaning toward thrombolytics.
Contraindications to thrombolytics: must be measured on the strength of the above indication.
Absolute contraindications: history of intracranial neoplasm or ICH, <2 months of intracranial trauma/injury/surgery, bleeding condition, ischemic CVA <3 months prior.
Disposition:
Perform PESI (PE Severity Index): this validated score adds patient’s age to points assigned to 10 variables. The total score organizes the patient to classes of increasing risk for mortality, with <66 being the least risk of mortality, and Class V >125 being the highest risk category.
sPESI is a more truncated approach but of similar accuracy.
Prognosis: risk of recurrence is highest within the 1st two weeks, declining thereafter. Mortality at 5 years is associated most with non-cardiovascular causes (infection, cancer, etc).
BONUS TIME! More on the protocols used. This is not an academic journal club discussion but a review of the evidence in support of our approach to PE workup.
Well’s Criteria: clinically validated. ~50% of physicians use this score incorrectly or not at all. Maybe due to poor application of the criteria, who knows.
Gestalt fairs well against the Wells Criteria and is often just as accurate, but data shows physicians tend to overestimate risks of PE. We suggest taking the time to calculate Wells in each patient whom PE is high on the working differential.
PERC Criteria: has been clinically validated. One crossover cluster-randomized noninferiority study tested 1916 ED’s and found PERC was not inferior to D-dimer in low risk patients. The application of PERC reduced ED stay (by at least 40 minutes), reduce CTPA usage (by nearly 10%), and only one patient was found to have an undiagnosed PE at 3 month follow up. PERC should not be used where the local population PE prevalence is >7%.
D-dimer: here’s the deal. This test is hotly debated. We believe it does have a role, but in a very select group of patients. The test is horribly specific (40-60%) with a high sensitivity. We support the use of age-adjusted cut-off values as age can reduce the specificity of the test.
Age (if over 50) x 10 = cutoff value in ng/mL
In low risk patients, <500 ng/mL effectively rules out PE. In moderate risk patients, handle this case-by-case à we favor CTPA in most. D-dimer should not be used in high risk patients.
References:
1. Helman, A, DeWit, K, Lang, E. Pulmonary Embolism Challenges in Diagnosis Part 1. Emergency Medicine Cases. August, 2018. https://emergencymedicinecases.com/pulmonary-embolism-challenges-diagnosis-part-1/. Accessed [date].
2. Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100:598.
3. Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med 2007; 120:871.
4. PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990; 263:2753.
5. Courtney DM, Sasser HC, Pincus CL, Kline JA. Pulseless electrical activity with witnessed arrest as a predictor of sudden death from massive pulmonary embolism in outpatients. Resuscitation 2001; 49:265.
6. Courtney DM, Kline JA. Prospective use of a clinical decision rule to identify pulmonary embolism as likely cause of outpatient cardiac arrest. Resuscitation 2005; 65:57.
7. Panos RJ, Barish RA, Whye DW Jr, Groleau G. The electrocardiographic manifestations of pulmonary embolism. J Emerg Med 1988; 6:301.
8. Geibel A, Zehender M, Kasper W, et al. Prognostic value of the ECG on admission in patients with acute major pulmonary embolism. Eur Respir J 2005; 25:843.
9. Ferrari E, Imbert A, Chevalier T, et al. The ECG in pulmonary embolism. Predictive value of negative T waves in precordial leads–80 case reports. Chest 1997; 111:537.
10. Shopp JD, Stewart LK, Emmett TW, Kline JA. Findings From 12-lead Electrocardiography That Predict Circulatory Shock From Pulmonary Embolism: Systematic Review and Meta-analysis. Acad Emerg Med 2015; 22:1127.
11. Worsley DF, Alavi A, Aronchick JM, et al. Chest radiographic findings in patients with acute pulmonary embolism: observations from the PIOPED Study. Radiology 1993; 189:133.
12. Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788.
13. Sista AK, Kuo WT, Schiebler M, Madoff DC. Stratification, Imaging, and Management of Acute Massive and Submassive Pulmonary Embolism. Radiology 2017; 284:5.
14. van Belle A, Büller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006; 295:172.
15. Klok FA, Mos IC, Nijkeuter M, et al. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Arch Intern Med 2008; 168:2131.
16. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of Patients With Suspected Acute Pulmonary Embolism: Best Practice Advice From the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med 2015; 163:701.
17. Bass AR, Fields KG, Goto R, et al. Clinical Decision Rules for Pulmonary Embolism in Hospitalized Patients: A Systematic Literature Review and Meta-analysis. Thromb Haemost 2017; 117:2176.
18. Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost 2008; 6:772.
19. Stein PD, Hull RD, Patel KC, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med 2004; 140:589.
20. Remy-Jardin M, Pistolesi M, Goodman LR, et al. Management of suspected acute pulmonary embolism in the era of CT angiography: a statement from the Fleischner Society. Radiology 2007; 245:315.



