Want to experience the greatest in board studying? Check out our interactive question bank podcast- the FIRST of its kind here: emrapidbombs.supercast.com
Author: Blake Briggs, MD
Peer Reviewer: Mary Claire O’Brien, MD
Objectives: review cardiac arrest and approach to ACLS, discuss ROSC, its goals; explain guidelines for hemodynamic support, discuss indications and contraindications for TTM and TH.
“Arrest” is another word for a sudden cessation in effective blood circulation due to cardiopulmonary failure. Arrest = death. If no medical intervention is performed these patients remain dead.
On average, sudden cardiac arrest is responsible for nearly 500,000 deaths annually. It is difficult to truly measure what actually causes patients to arrest.
See our handout on our website, “Shot through the heart: sudden cardiac arrest and its causes”
Guidelines have been shifting for several decades now. Advanced Cardiac Life Support (ACLS) are clinical management guidelines for various life-threatening conditions. It is the standard for emergent healthcare delivery in North America. It provides the rubric (or “playbook”) for managing sudden cardiac arrest, colloquially known as “running a code”.
Despite its portrayal in Hollywood, the majority of patients do not reach hospital discharge, and even fewer survive with good neurologic outcome. As research continues, clinicians should focus on what has been shown to provide the best possible chance at survival with good neurologic outcome.
Accelerate your learning with our EM Question Bank Podcast
- Rapid learning
- Interactive questions and answers
- new episodes every week
- Become a valuable supporter
The most critical actions to perform in running a code:
1) Excellent CPR
2) Defibrillation as soon as possible (if indicated for shockable rhythms)
Without CPR and defibrillation, survival from cardiac arrest caused by VF declines by ~10% for each minute, and after more than 12 minutes without CPR, the survival rate is <5%. Survival to hospital discharge for those treated between 1998 and 2001 has not significantly changed, and this is most likely due to delayed CPR onset and/or poor CPR in general. It cannot be understated how CPR education needs to be a priority.
Running a code
Patient enters cardiac arrest —> not breathing + no pulse. CPR needs to be started immediately. Studies have repeatedly shown that the sooner CPR begins, the better chance of survival. If you do not feel a pulse, YOU start CPR. Do not call for others to come start CPR. YOU DO. It is the single, most important intervention. This is so incredibly high yield for boards and life, it cannot be overstated. If you are in a hospital, begin CPR and alert someone. If you are not in a healthcare setting, begin CPR and tell someone nearby to call 911. If you are alone, perform CPR while yelling for help and if you are still alone after 2 minutes quickly look for help and secure an AED if nearby. The AED should be turned on and pads placed. While charging the defibrillator, continue CPR. The time from pulse check to hitting “shock” on the defibrillator should not take more than 5 seconds.
Let’s proceed with the basic overall approach of “running a code”:
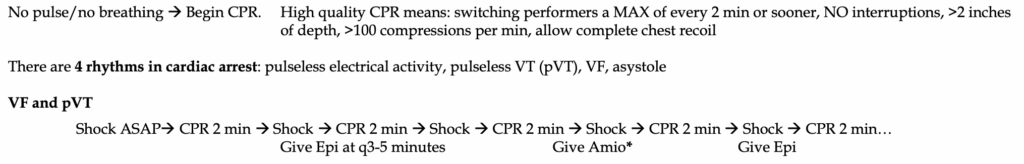
-Key points: defibrillation is ONLY used in pVT and VF. Decreased time to defibrillation improves the likelihood of successful conversion to a perfusing rhythm. If a patient sustains witnessed VF or pVT arrest and a defibrillator is immediately available and pads are in place, immediately charge the defibrillator and deliver a shock. The ten seconds or fewer of CPR are unlikely to generate any substantive perfusion.
First dose of epinephrine should be given during the 2nd round of CPR and given from then on, every 3-5 minutes. After the third round of epinephrine, give a dose of 300 mg Amiodarone instead, followed by a 2nd dose of 150mg 2 rounds later. In summary, you should be alternating amiodarone and epinephrine every 3-5 minutes along with defibrillation when indicated on the monitor.
The success of defibrillation is directly dependent on the duration of VF/VT and speed to initiate defibrillation. Pulse checks should take <5 seconds in between CPR breaks and should NOT occur after shock delivery. Immediately resume CPR.
*Lidocaine has been found to be no different in outcomes than Amiodarone and can be given in place of it.
See our podcast on iTunes/Soundcloud- “Episode 42. Precious Joules: Cardioversion in the ED”
-Key points: NO defibrillation should be performed in PEA/asystole. No evidence supports its use. The patient is suffering from some nonelectrical pathology that is impairing cardiac muscle contractility. This is where we remember those dreaded H’s and T’s:

When do I deliver ventilations?
The ratio of 30:2 (CPR to ventilation) has long been preferred but if no bag is available, straight CPR takes precedence until help arrives. Giving “mouth-to-mouth” breathes has fallen out of favor. If the patient has an advanced airway, deliver ventilations at 6-8/minute.
End-tidal CO2 should be utilized during CPR to reflect cardiac output and cerebral perfusion. Decreased EtCO2 suggests poor compressions and/or poor prognosis.
In children, it has been found 30:2 and 15:2 ratios are preferred since the #1 cause of arrest in children is respiratory.
When do I intubate?
Despite popular belief, endotracheal intubation is NOT a priority during CPR. In fact, studies support ventilations via bag-valve-mask. Airway adjuncts are encouraged such as an LMA or other supraglottic airway.
Endotracheal intubation can drastically drop BP and create more problems. Also, intubating during CPR is difficult and halting compressions for 5-10 seconds is undesirable.
If a dedicated, experienced airway physician is available and CPR is unhindered, a supraglottic airway or ETT can be placed and may improve ventilatory effort.
Where’s all the evidence? Since survival rates are abysmal, and solid evidence is difficult to come by, the most controversial being medications. As of this writing, a growing body of evidence raises doubt over the effectiveness of epinephrine and its possibly deleterious effects during resuscitation. Several studies have shown epinephrine does not improve survival to hospital discharge. Other studies suggest epinephrine can worsen neurologic outcomes. Maybe the circulating catecholamines cause a negative effect on brain perfusion leading to worse neurologic outcomes. Maybe epinephrine needs to be at lower and less frequent dosing. Maybe we shouldn’t be using epinephrine at all. As of now, unless clinical gestalt dictates overwise, you should refer to published ACLS guidelines as defined above- THAT is what is being tested. In real life, you don’t necessarily have to give epinephrine every 3-5 minutes- we sure don’t; however, this is advanced level thinking among experienced providers.
Amiodarone/lidocaine has been found to provide little survival benefit as well and does not result in increased survival to hospital discharge from several studies.
When to terminate efforts: little data published to guide decision-making. The following factors strongly suggest halting efforts:
-duration of resuscitation >30 minutes, initial rhythm of asystole/PEA, unwitnessed arrest, prolonged time between arrest and initiation of CPR, low EtCO2 even after 20 minutes of high-quality resuscitation.
-bedside echo showing no cardiac wall motion. This finding alone should never terminate resuscitation, and it should never interfere with CPR.
Therapies that should never be done: Atropine (no role in PEA/asystole), vasopressin, cardiac pacing for asystole or PEA.
Return of spontaneous circulation (ROSC):
Once pulses have been achieved, every possible attempt should be made at making sure you don’t lose them again. At the same time, there should be a vigorous investigation into what was the cause of arrest.
Critical objectives during ROSC:
-managing hemodynamics post-arrest
-minimizing brain injury
-securing a definitive, endotracheal airway if not done so already
-diagnose and treat suspected causes of arrest
Immediately, an EKG should be performed. The #1 cause of sudden cardiac arrest worldwide is MI. MI’s go to the cath lab.
Reassess the patient post-arrest. What did you miss while you were running the resuscitation? Rigid abdomen? Loud, harsh cardiac murmur? Decreased lung sounds unilaterally? Tachycardia is expected after ROSC, but bradycardia is ominous. Blood in the OG tube or rectum suggests GI bleeding and warrants emergency release blood products, unilateral leg swelling suggests PE. Track marks on the skin suggest IVDA which in turn place the patient at risk for endocarditis and septic emboli.
Tests you should order: EKG #1, CXR, CT head. Bedside echocardiography should be encouraged.
Other studies: Blood gas (because, why not), CMP, CBC, serial troponins, lactate, +/- others depending on patient presentation.
Any evidence of STEMI requires emergent reperfusion therapy. Even in those without STEMI but had VF or pVT arrest might also benefit. In any case, cardiology should be consulted after ROSC in all patients who suffered VF/VT arrest.
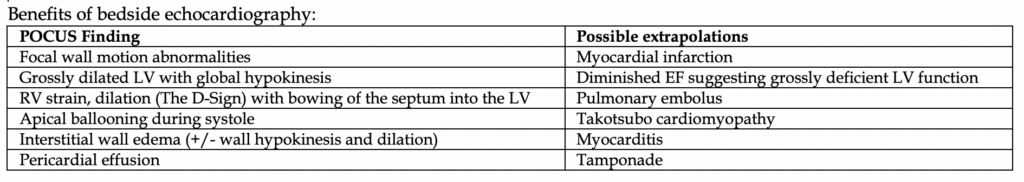
You should also conduct a neurologic evaluation. Patients who cannot perform any purposeful movements on basic command meet indication for therapeutic hypothermia and targeted temperature management (TTM).
Hemodynamic support:
Cardiovascular
Maintain MAP goal >65 mmHg, preferably 80-100 to prevent cerebral vasoconstriction.
-IV fluids can assist in maintain a normal CVP ~8-12. Lactated Ringers should be the fluid of choice.
-There is no specific guideline or standardized research that clearly shows one vasopressor or inotropic agent that is superior to another in these patients.
Commonly used agents: epinephrine, norepinephrine, dopamine.
There has been a gradual shift towards preferring norepinephrine in these patients, although the data is incomplete.
In terms of inotropic agents, dopamine or milrinone has been used. Both cause hypotension; dopamine predisposes patients to tachyarrhythmias.
Respiratory
-It’s now time to intubate if you haven’t. Optimize BP first though, as the most common post-intubation complication is hypotension.
-Maintain normal ventilation (PaCO2 35-40), Normoxia >94% (not hyperoxia which can have worse outcomes).
-Monitor closely with end-tidal CO2
Neurologic
Fever = worse neurologic outcomes. It is the most common cause of death in patients with cardiac arrest outside the hospital.
32-36 C improves outcome. (89-93 F).
Targeted temperature management (TTM) refers to temperature control no higher than 36 Celsius. Therapeutic hypothermia (TH) refers to active range maintenance of 32-34 Celsius.
-Indications of TTM: any patient not following commands or having purposeful movements following any cardiac arrest. Specifically, patients who have suffered from VF or pVT seem to respond better and have greater chances of improved neurologic outcomes. Discussion of initiating TTM and TH needs to occur with the intensivist in the correct setting, as they will be the ones managing the patient’s care much longer than the ED.
-Contraindications of TTM: no absolute contraindications. Relative contraindications include cardiogenic shock, uncontrolled bleeding
-Duration: 24-48 hours
-Methods: infuse cold saline, >2 C per hour (1 L in 15 min can lower 1 C). Surface blankets work too. Do not give fluids in those with ESRD/CHF. Instead, prefer blankets.
-Side effects: shivering (sedate patient) poor coagulation, increased infection risk.
-Serial blood gases should be performed as ventilation requirements will decrease as temperature decreases. The goal PaCO2 is 35-40.
References
1. Ali MU, Fitzpatrick-Lewis D, Kenny M, et al. Effectiveness of antiarrhythmic drugs for shockable cardiac arrest: A systematic review. Resuscitation 2018; 132:63.
2. Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002; 346:557.
3. Donnino MW, Rittenberger JC, Gaieski D, et al. The development and implementation of cardiac arrest centers. Resuscitation 2011; 82:974.
4. Eftestøl T, Sunde K, Steen PA. Effects of interrupting precordial compressions on the calculated probability of defibrillation success during out-of-hospital cardiac arrest. Circulation 2002; 105:2270.
5. Gaieski DF, Band RA, Abella BS, et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation 2009; 80:418.
6. Gebhardt K, Guyette FX, Doshi AA, et al. Prevalence and effect of fever on outcome following resuscitation from cardiac arrest. Resuscitation 2013; 84:1062.
7. Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002; 346:549.
8. Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med 2004; 30:2126.
9. Link MS, Berkow LC, Kudenchuk PJ, et al. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015; 132:S444.
10. Kim F, Olsufka M, Longstreth WT Jr, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation 2007; 115:3064.
11. Metter RB, Rittenberger JC, Guyette FX, Callaway CW. Association between a quantitative CT scan measure of brain edema and outcome after cardiac arrest. Resuscitation 2011; 82:1180.
12. McKenzie N, Williams TA, Tohira H, et al. A systematic review and meta-analysis of the association between arterial carbon dioxide tension and outcomes after cardiac arrest. Resuscitation 2017; 111:116.
13. Neumar RW, Otto CW, Link MS, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122:S729.
14. Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med 2013; 369:2197.
15. Panchal AR, Berg KM, Kudenchuk PJ, et al. 2018 American Heart Association Focused Update on Advanced Cardiovascular Life Support Use of Antiarrhythmic Drugs During and Immediately After Cardiac Arrest: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2018; 138:e740.
16. Rea TD, Eisenberg MS, Becker LJ, et al. Temporal trends in sudden cardiac arrest: a 25-year emergency medical services perspective. Circulation 2003; 107:2780.
17. Reynolds JC, Callaway CW, El Khoudary SR, et al. Coronary angiography predicts improved outcome following cardiac arrest: propensity-adjusted analysis. J Intensive Care Med 2009; 24:179.
18. Rittenberger JC, Tisherman SA, Holm MB, et al. An early, novel illness severity score to predict outcome after cardiac arrest. Resuscitation 2011; 82:1399.
19. Rudolf J, Ghaemi M, Ghaemi M, et al. Cerebral glucose metabolism in acute and persistent vegetative state. J Neurosurg Anesthesiol 1999; 11:17.
20. Schaafsma A, de Jong BM, Bams JL, et al. Cerebral perfusion and metabolism in resuscitated patients with severe post-hypoxic encephalopathy. J Neurol Sci 2003; 210:23
21. Soar J, Donnino MW, Maconochie I, et al. 2018 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations Summary. Resuscitation 2018; 133:194.
22. Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016; 133:e38.
23. Winkle RA, Mead RH, Ruder MA, et al. Effect of duration of ventricular fibrillation on defibrillation efficacy in humans. Circulation 1990; 81:1477.
24. Yu T, Weil MH, Tang W, et al. Adverse outcomes of interrupted precordial compression during automated defibrillation. Circulation 2002; 106:368.
25. Zeiner A, Holzer M, Sterz F, et al. Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Arch Intern Med 2001; 161:2007.



