Want to experience the greatest in board studying? Check out our interactive question bank podcast- the FIRST of its kind here: emrapidbombs.supercast.com
Author: Blake Briggs, MD
Peer editors: Travis Smith, DO, Mary Claire O’Brien, MD
Introduction
Myasthenia gravis is a chronic autoimmune neuromuscular disease with an incidence of ~14-40 cases per 100,000 annually in the United States.1-4 It is classically tested in medical school, however, it can be easily mismanaged in the acute clinical setting. The hallmark is a variation in ocular, bulbar, and limb motor weakness, secondary to T-cell mediated attack on postsynaptic membrane acetylcholine receptors. It is important to denote that there are two forms of the disease, ocular myasthenia which is limited to the eyes, and generalized myasthenia. For the purposes of this document, we will focus on the generalized condition.
Clinical presentation
It can occur at any age but has a bimodal distribution of onset, with a classic peak in the 20-30s in females (typical of many autoimmune diseases).5
The most classic finding is true muscle fatigue, which worsens with increased use of that muscle. Fatigue without weakness is not myasthenia gravis and we know it best as asthenia. Importantly, patients with myasthenia have specific, tangible weakness from muscle usage, not “generalized weakness”.
The weakness fluctuates as the patient uses some muscles more than others throughout the day.6 This is also important as it differentiates from other neuromuscular and motor neuron diseases where the weakness is constant.
Myasthenia can affect any skeletal muscle, but there are some that are easier to observe than others, hence ptosis.
Ptosis is seen in >50% of patients, along with diplopia or other ocular symptoms like weakness of extraocular eye movements.7-9 In those with isolated ocular findings, >50% will have generalized myasthenia in <2 years.
<5% present with isolated arm or leg weakness. Arms >> legs, with proximal muscles >> distal ones.
Accelerate your learning with our EM Question Bank Podcast
- Rapid learning
- Interactive questions and answers
- new episodes every week
- Become a valuable supporter
15% have bulbar symptoms. This typically includes jaw weakness, especially with chewing, and is noted about halfway through eating.10 Dysarthria and dysphagia may also occur. The patient’s speech may sound as though it is low intensity (hypophonic) or nasal due to muscle weakness.
Weakness of the neck muscles can cause the head to droop as well.
Respiratory muscle fatigue is the most concerning symptom of myasthenia gravis. The respiratory failure causes a “myasthenic crisis” and can progress slowly or rapidly during the patient’s presentation with or without other symptoms.
High-risk medications
There are countless medications that can exacerbate myasthenia gravis and should be avoided. We do not expect anyone to memorize this list, but here are a few medications that should never be given.
Neuromuscular blockers (obvious risk vs benefit analysis. Should only be used if emergent intubation is necessary)
Aminoglycosides
Fluoroquinolones
Macrolides
Beta-blockers
Procainamide
Aminoglycosides
Quinidine
Chloroquine
Hydroxychloroquine
Symptoms early in the course of the disease may be transient, but slowly progressive, peaking in about 1 year. In fact, many patients have about 5 years of intermittent symptoms marked by multiple crisis episodes, followed by a stable period for multiple years and eventually remission.7
Diagnosis & Treatment
This goes way beyond the ED, as electrophysiologic studies and autoantibody testing are needed. The ice pack test is helpful at the bedside for patients with ptosis or in those with symptoms of diplopia. Basically, neuromuscular transmission is better at lower temperatures, so by applying an ice pack for 2 minutes to orbits, ptosis should immediately improve once the ice pack is removed. The test is 80% sensitive, but its predictive value has never been assessed.11
Edrophonium is no longer available in the US so there’s no point in discussing this.
Patients having symptomatology consistent with myasthenia gravis should all be admitted for an expedited workup. It is difficult to predict when respiratory symptoms occur but sending them home is a bad option.
Long-term treatment of myasthenia gravis is aimed at chronic immunosuppressants, symptomatic therapy in the form of acetylcholinesterase inhibitors (pyridostigmine), and rapid, short-acting immunomodulators (IVIG). Thymectomy is occasionally needed for patients given the association of thymomas with myasthenia (15% of patients) and chest imaging is usually warranted to rule out its presence.
Myasthenic Crisis
As an emergency physician, you are unlikely to outright diagnose myasthenia gravis, but you are much more likely to manage a myasthenic crisis.
A myasthenic crisis is defined as worsening weakness leading to respiratory depression and eventual intubation or NIPPV.1 ~20% of patients will experience a crisis at least once in their disease course.12 In fact, in ~20%, a crisis is the initial presentation of myasthenia.13
Rapid therapies for those in myasthenic crisis include plasma exchange and IVIG. These start to have effect in several days, and therefore do not play a role in management in the ED and are typically started in the ICU.14
Your only job in the ED is to admit the patient to the ICU and discuss elective intubation. Elective intubation is much preferred over “urgent” or “emergent,” as the procedure is more controlled, and outcomes are better.15
Classically, patients will present with worsening bulbar and generalized weakness. Unfortunately, the “generalized” weakness can hide respiratory fatigue and distract the clinician. Even worse, respiratory muscles can suddenly worsen.
The most common cause of a crisis is an infection or some physiological stressor like pregnancy, recent surgery, tapering or missing medication doses, and taking drugs that exacerbate myasthenia (see above).13
The most critical aspect of care in the emergency department is an assessment of their respiratory function. All patients with suspected myasthenic crisis require ICU admission.
Clinical signs that alert you to impending respiratory failure include:
● Dyspnea upon lying down: this isn’t just simple shortness of breath. This is a scary feeling of “drowning” and an inability to take a deep breath when lying down due to diaphragm fatigue.
● Any bulbar symptoms: difficulty clearing secretions, dysarthria (“muffled voice”), weak cough.
● Late findings include poor respiratory effort: hypophonia, only speaking a few words before taking a deep breath, shallow breaths, use of accessory muscles during inspiration. If any of these are seen, you are very close to impending respiratory failure.
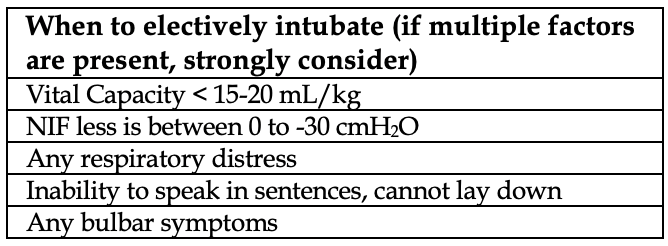
“Objective” measures of respiratory failure can help identify who is at higher risk of impending failure and necessitating elective intubation, but we put quotes over the word objective because these values must be taken in context with the clinical presentation of the patient.
Vital Capacity: reflects inspiratory and expiratory function and can be performed at the bedside by a respiratory therapist. The patient is placed in a sitting position and inhales deeply, followed by a maximal exhalation into a spirometer. A value below 15-20 mL/kg is considered predictive of severe respiratory fatigue.
MIP (or NIF, negative inspiratory force): only provides information on inspiratory strength. The patient inhales against a closed valve from a resting tidal volume. Inspiration against a closed valve generates a negative force, so the values are negative numbers. A NIF <1/3 of normal (0 to -30 cmH2O) predicts a probable deterioration. The decision to intubate is based on a variety of factors and must all be taken within the clinical context as noted in the above table.
NIPPV may be utilized on a case-by-case basis and has been shown to decrease the rate of intubation in 38% of patients in one study (92 patients).16 Obviously, NIPPV is inappropriate in patients who are too weak to participate, especially those with severe bulbar symptoms. We believe there needs to be a very low threshold to intubate, and do not rely on NIPPV in patients with concerning symptoms for progressive respiratory failure.
Prognosis
Myasthenic crises are associated with in-hospital mortality of ~10%, even with neurointensive care.16 The most common causes of death are sepsis and respiratory failure. ~50% will need some form of rehabilitation upon discharge.
References
1. Carr AS, Cardwell CR, McCarron PO, McConville J. A systematic review of population based epidemiological studies in Myasthenia Gravis. BMC Neurol 2010; 10:46.
2. Heldal AT, Eide GE, Gilhus NE, Romi F. Geographical distribution of a seropositive myasthenia gravis population. Muscle Nerve 2012; 45:815.
3. Mombaur B, Lesosky MR, Liebenberg L, et al. Incidence of acetylcholine receptor-antibody-positive myasthenia gravis in South Africa. Muscle Nerve 2015; 51:533.
4. Breiner A, Widdifield J, Katzberg HD, et al. Epidemiology of myasthenia gravis in Ontario, Canada. Neuromuscul Disord 2016; 26:41.
5. Boldingh MI, Maniaol AH, Brunborg C, et al. Increased risk for clinical onset of myasthenia gravis during the postpartum period. Neurology 2016; 87:2139.
6. Nicolle MW. Myasthenia Gravis and Lambert-Eaton Myasthenic Syndrome. Continuum (Minneap Minn) 2016; 22:1978.
7. Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve 2008; 37:141.
8. Grob D, Arsura EL, Brunner NG, Namba T. The course of myasthenia gravis and therapies affecting outcome. Ann N Y Acad Sci 1987; 505:472.
9. Oosterhuis HJ. The natural course of myasthenia gravis: a long term follow up study. J Neurol Neurosurg Psychiatry 1989; 52:1121.
10. Pal S, Sanyal D. Jaw muscle weakness: a differential indicator of neuromuscular weakness–preliminary observations. Muscle Nerve 2011; 43:807.
11. Benatar M. A systematic review of diagnostic studies in myasthenia gravis. Neuromuscul Disord 2006; 16:459.
12. Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist 2011; 1:16.
13. Berrouschot J, Baumann I, Kalischewski P, et al. Therapy of myasthenic crisis. Crit Care Med 1997; 25:1228.
14. Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: Executive summary. Neurology 2016; 87:419.
15. Juel VC. Myasthenia gravis: management of myasthenic crisis and perioperative care. Semin Neurol 2004; 24:75.
16. Neumann B, Angstwurm K, Mergenthaler P, et al. Myasthenic crisis demanding mechanical ventilation: A multicenter analysis of 250 cases. Neurology 2020; 94:e299.
17. National Organization for Rare Disorders. Myasthenia Gravis. https://rarediseases.org/rare-diseases/myastheniagravis/#affected-populations.



