Want to experience the greatest in board studying? Check out our interactive question bank podcast- the FIRST of its kind here: emrapidbombs.supercast.com
Authors: Blake Briggs, MD; Travis Smith, DO
Introduction
Ischemic stroke describes a large group of conditions which result in decreased or complete obstruction of blood flow to brain tissue. It is very common, major source of morbidity and mortality. In the US alone, the lifetime risk for all adults is 25%. The annual incidence is around 800,000. Blacks and Hispanics have a higher risk compared to whites. Ischemic strokes make up 80% of all stroke with the most common cause being thrombosis, followed by embolism, and then systemic hypoperfusion. This document will not cover hemorrhagic stroke (20% of strokes). We will also not cover the specifics of “where is the lesion” when it comes to diagnosing where the particular stroke is. The latter is not high yield for boards and does not typically change management in the ED. Your role as an emergency provider is to recognize stroke and successfully navigate the field of diagnosis and stabilization. tPA, ever the controversial topic, will not be debated here. We have our own opinions on tPA, but our job is to teach what you need to know for practice and the boards. In practice you will have patients getting tPA, and until that changes, it is standard of care in the US and is board testable.
Strokes are all about classification, location, vessel, and deficits noted. This is critical in determining treatment pathways.
Thrombosis: in situ obstruction of an artery results in low blood flow. These can be divided into large or small vessel disease.
· Large vessel disease: extracranial arteries (external and internal carotids, vertebral), and the Circle of Willis with its associated proximal main branches.
What causes obstruction? The stuff we learned in medical school- atherosclerosis (#1 cause by far), dissection (see our handout on cervical dissection), arteritis/vasculitis, Moyamoya syndrome, fibromuscular dysplasia, and vasoconstriction.
· Small vessel disease: intracerebral penetrating arteries that arise from the distal vertebral artery, basilar artery, and middle cerebral artery. The most common areas affected by these vessels are “deep brain tissues” (e.g. basal ganglia, internal capsule, thalamus, pons). These strokes are coined lacunar infarcts.
Embolism: symptoms are abrupt and maximal at the start due to the sudden blockage of a vessel from a clot. The most common source is cardiac.
Accelerate your learning with our EM Question Bank Podcast
- Rapid learning
- Interactive questions and answers
- new episodes every week
- Become a valuable supporter
Systemic hypoperfusion: most commonly found in shock conditions where there is reduced cardiac output or cardiac arrest. Symptoms are classically diffuse and non-focal. The “watershed” regions are most at risk for severe ischemia. These are areas between cerebral blood supplies and are quite vulnerable to hypoperfusion.
Initial Assessment and Management
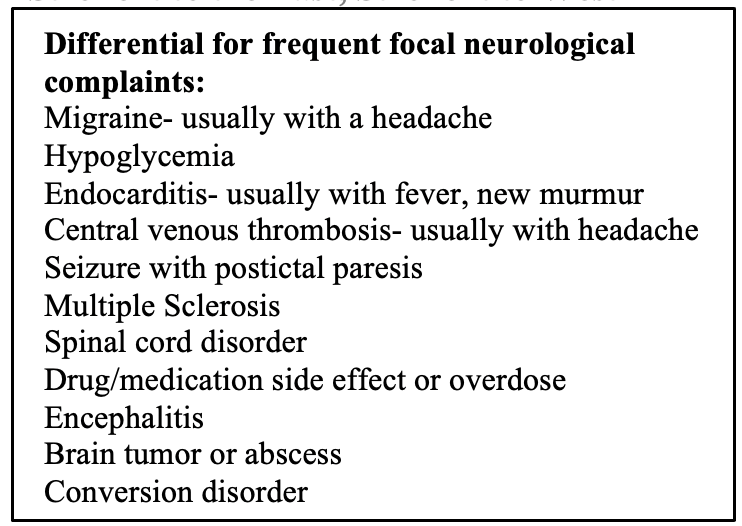
The immediate goal of stroke assessment in the ED is to minimize brain injury and treat medical complications. At the same time, you must have an open mind and large differential as there are a great number of stroke mimickers.

Time is brain as they say. ABC assessment, history, neurological exam, fingerstick glucose, and a set of vitals should take place within 3 minutes, no more.
Those with decreased consciousness, bulbar deficits, or associated seizures may not be good candidates for laying down in a CT scanner and might require immediate intubation. This is more typical for hemorrhage stroke patients (typically have higher blood pressures on initial presentation).
The most critical aspect of the history is the time of onset. Time will determine eligibility for IV thrombolysis (tPA; <4.5 hours) and endovascular
thrombectomy (EVT; <24 hours).
If patients are unable to reliably provide this, symptom onset is defined as the “last known normal” for baseline function. Family, friends, and EMS should help with this (imaging with CT perfusion can help identify those with salvageable brain tissue that still qualify for endovascular thrombectomy or tPA).
Any other historical questions are good to ask if the patient is able to answer them but do not let this delay neuroimaging.
The physical exam should be concise and efficient. There are many scales available, the NIHSS (comprised of 11 items), is the most popular and ranges from 0 to 42 (<5 for mild, 5-9 for moderate, >10 for severe). The NIHSS has been correlated to stroke outcomes, although admittedly it is less effective at capturing posterior circulation strokes.
The most predictive symptoms for stroke are facial paresis, arm drift/weakness, and abnormal speech (either dysarthria or language comprehension).
CT head and CTA should be performed.
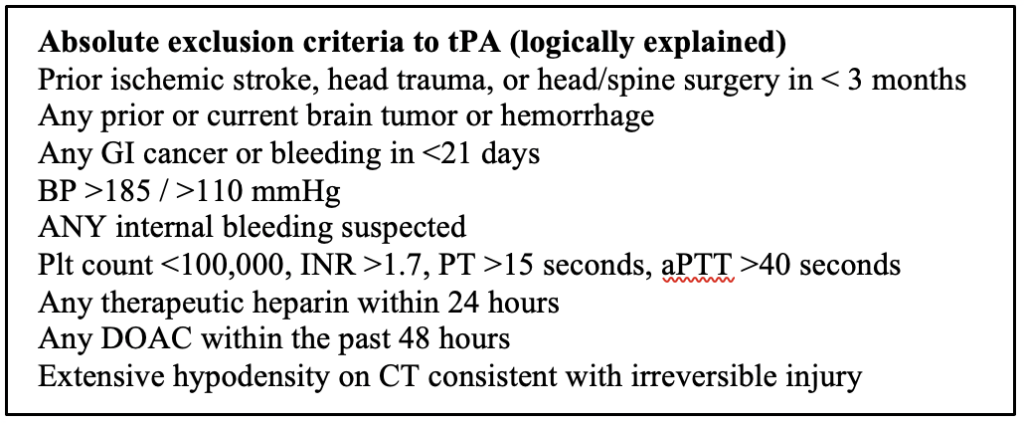
The other usual studies include EKG, CBC, BMP, and coagulation studies. Notably, tPA should not be routinely delayed awaiting coagulation study results. In other words, in the majority of cases, the only 2 tests that are required before administering tPA is a fingerstick glucose and a CT head.
The only time you should wait for the coagulation studies if there is a suspected bleeding abnormality or patient use of heparin or warfarin.
Other lab studies can be added if there is a concern for other diagnoses (see table below for stroke mimickers). For example, blood cultures, ESR, CRP, urine studies, and troponins should be added in those where endocarditis or some other infectious etiology is a concern.
Stroke can cause EKG changes; this is especially true of subarachnoid hemorrhages. EKGs with evidence of new-onset atrial fibrillation suggest an embolic stroke etiology.
Stroke management in the ED
Discuss if the patient is a candidate for tPA and/or EVT (see tables).
IV fluids: in those who appear dehydrated, volume depletion worsens cerebral blood flow.
Hypoglycemia: should be immediately corrected if < 60 mg/dL. Hyperglycemia: likely a component of stress and uncontrolled baseline hyperglycemia. If >180 mg/dL it should be lowered as it is associated with worsening brain injury. Remember that hyperglycemia can cause a transient worsening of poststroke neurologic deficits or reemergence of previous stroke-related deficits (or poststroke recrudescence [PSR]). However, tight glucose control with IV insulin has NOT been associated with improved functional outcomes. Be judicious with insulin as hypoglycemia is a serious complication.
Blood pressure: see our handout on hypertensive emergency for details and agent preference. There are key differences in hemorrhagic vs ischemic stroke management. To be clear, there’s a lot of debate, but we will spare you the details and tell you what’s considered “cool” right now:
– If eligible for tPA, blood pressure should be targeted at <185 mmHg systolic and <110 mmHg diastolic. The BP should be around 180/105 for at least 24 hours after thrombolytic therapy. DO NOT rapidly lower the BP as it is okay for it to be around 180s-170s due to cerebral autoregulation and distal vessel dilation.
– If not eligible for tPA, blood pressure should be targeted at <220 mmHg systolic or diastolic <120.
Acute stroke therapy
tPA: Let’s talk about the elephant in the room, does it work or not? Watch this debate of two EM juggernauts and you decide. It is estimated that 2-5% of all strokes will get tPA. Alteplase is first-line therapy, dosed at 0.9/mg/kg. You must confirm the following prior to tPA administration: persistent neurologic deficit on exam that is considered disabling (i.e. prevents basic daily life functions), serum glucose is normal, BP goals are met, >1 large-bore IV line is present, all eligibility criteria are met. 10% of the dose is given over 1 minute as a bolus and the rest is given over one hour.

What about those with an unwitnessed stroke or the “wake-up” stroke >4.5 hours symptom onset? There is mixed data, but promising, that suggests tPA might be of benefit. Here, stat MRI is performed, and they look for an ischemic parenchymal brain lesion on DWI. None of this will be on your boards as it is still controversial and not established therapy.
tPA is controversial, and we are not going to even try to debate the pro’s/cons of it. What you need to know is that tPA is standard of care at this time, and it will be on your exams.
tPA complications
All patients who receive tPA get admitted to the ICU for 24 hours of monitoring. Any decline in neurologic function, new headache, nausea/vomiting, or sudden rise in BP necessitates a stat CT head to rule out intraparenchymal hemorrhage. It is estimated that major systemic hemorrhage, and angioedema occur in approximately 2%, and 5% of patients, respectively.
In those with symptomatic intracranial hemorrhage (sICH), the mortality is quoted as high as 82% (no wonder why we all have concerns about who we give this medication too).
However, risk of sICH increases with certain factors: baseline NIHSS >20, Age >70, Ischemic changes on initial CT, glucose >300. If there are none of these risk factors present, risk of sICH is 2%. 1 risk factor present, 5% risk, >1 risk factor present, 22% risk.
There is no proven reversal strategy for tPA, but the following are a few treatment options: cryoprecipitate, antifibrinolytic agents, prothrombin complex concentrates, TXA, platelets if count <100,000. FFP and protamine sulfate can be given if the patient was previously on warfarin or heparin.
Bleeding gums, oozing from the IV site, and ecchymoses are common and don’t require tPA cessation. Pericardial effusion and serious GI bleeding require cessation.
EVT: mechanical thrombectomy is indicated for patients with acute ischemic stroke due to large vessel occlusion in the anterior circulation, regardless if they received alteplase or not. EVT might have a role in posterior circulation strokes, but trials excluded these patients initially.
References
- Caplan LR. Intracranial branch atheromatous disease: a neglected, understudied, and underused concept. Neurology 1989; 39:1246.
- GBD 2016 Lifetime Risk of Stroke Collaborators, Feigin VL, Nguyen G, et al. Global, Regional, and Country-Specific Lifetime Risks of Stroke, 1990 and 2016. N Engl J Med 2018; 379:2429.
- Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020; 141:e139.
- White H, Boden-Albala B, Wang C, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 2005; 111:1327.
- Doufekias E, Segal AZ, Kizer JR. Cardiogenic and aortogenic brain embolism. J Am Coll Cardiol 2008; 51:1049.
- Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific Rationale for the Inclusion and Exclusion Criteria for Intravenous Alteplase in Acute Ischemic Stroke: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2016; 47:581.
- Kothari R, Hall K, Brott T, Broderick J. Early stroke recognition: developing an out-of-hospital NIH Stroke Scale. Acad Emerg Med 1997; 4:986.
- Generalized efficacy of t-PA for acute stroke. Subgroup analysis of the NINDS t-PA Stroke Trial. Stroke 1997; 28:2119.
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019; 50:e344.
- Burns JD, Green DM, Metivier K, DeFusco C. Intensive care management of acute ischemic stroke. Emerg Med Clin North Am 2012; 30:713.
- Johnston KC, Bruno A, Pauls Q, et al. Intensive vs Standard Treatment of Hyperglycemia and Functional Outcome in Patients With Acute Ischemic Stroke: The SHINE Randomized Clinical Trial. JAMA 2019; 322:326.
- Yamaguchi T, Mori E, Minematsu K, et al. Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan Alteplase Clinical Trial (J-ACT). Stroke 2006; 37:1810.
- Michaels AD, Spinler SA, Leeper B, et al. Medication errors in acute cardiovascular and stroke patients: a scientific statement from the American Heart Association. Circulation 2010; 121:1664.
- Hill MD, Buchan AM, Canadian Alteplase for Stroke Effectiveness Study (CASES) Investigators. Thrombolysis for acute ischemic stroke: results of the Canadian Alteplase for Stroke Effectiveness Study. CMAJ 2005; 172:1307.
- Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014; 384:1929.
- Mazya M, Egido JA, Ford GA, et al. Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: safe Implementation of Treatments in Stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke 2012; 43:1524.
- Ambrosioni J, Urra X, Hernández-Meneses M, et al. Mechanical Thrombectomy for Acute Ischemic Stroke Secondary to Infective Endocarditis. Clin Infect Dis 2018; 66:1286.



