Want to experience the greatest in board studying? Check out our interactive question bank podcast- the FIRST of its kind here: emrapidbombs.supercast.com
Author: Blake Briggs MD
Peer Reviewer: Travis Smith, D.O.
Introduction
Intracranial hemorrhage is the second most common cause of all strokes, with ischemic stroke being first. This review will cover the practical diagnosis and management of ICH in the ED.
Risk factors
There are lots of underlying pathologies causing an intracranial bleed. The most common underlying pathology overall is hypertensive vasculopathy. Others that come up include ruptured saccular aneurysm, AV malformations, sympathomimetic abuse, tumors, Moyamoya disease, cerebral venous thrombosis, TPA, and even amyloid.1 For the record, AV malformations are the most common cause in children.2
Risk factors: hypertension, anticoagulant usage (especially warfarin)
Lower risk with the DOACs (direct oral anticoagulants)
Antiplatelets: small absolute increased risk with aspirin or others, but this has been debated.3
Accelerate your learning with our EM Question Bank Podcast
- Rapid learning
- Interactive questions and answers
- new episodes every week
- Become a valuable supporter
NSAIDs have not been found to increase risk.4
Pathophysiology of brain injury
Intracranial hemorrhages are so bad because of the limited, fixed volume of the skull. As the clot expands, there is increasing cytotoxic damage and edema, worsening the mass effect and increased intracranial pressure, furthering the risk of herniation.5
In the majority of patients, the bulk of hemorrhage expansion occurs in the first 3 hours after onset.6
Expansion into the intraventricular space occurs in nearly 50% of patients and is associated with worse outcomes.7
The greatest predictor of hemorrhage growth is anticoagulant use, contrast extravasation (Spot Sign), and ICH volume on baseline imaging.8
Obviously, hemorrhage enlargement is bad and clearly is associated with neurologic deterioration and worse outcomes.
So, improvements in patient outcomes from ICH may be achieved by minimizing both secondary brain ischemia and hemorrhage enlargement in the early hours following the onset of bleeding. We will cover this in the management section.
Presentation
Most cases occur during routine activity. Neurologic symptoms may develop rapidly in minutes or hours.
Common associated symptoms include headache and vomiting, together seen in over 50% of patients. Decreased level of consciousness can occur if the hemorrhage is large enough.
Small hemorrhages rarely cause headaches. They are more likely to cause progressive stroke symptoms.
Coma/stupor is an ominous sign, with the one exception being thalamic hemorrhage. Those patients may recover after blood is reabsorbed from the reticular activating system.
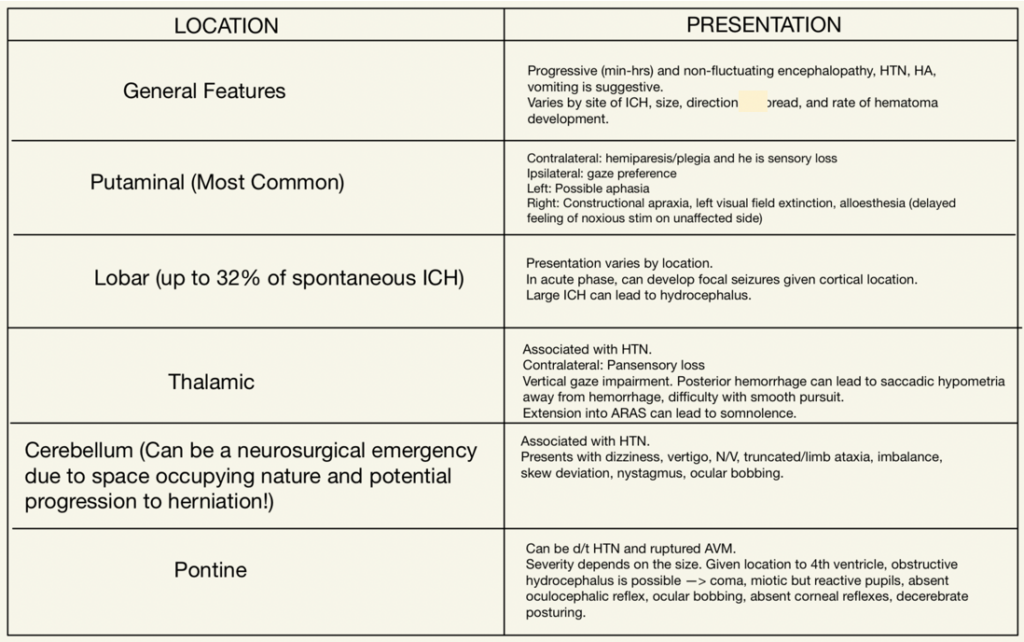
Neurologic symptoms and signs greatly depend on location, much like an ischemic stroke. You can expect varying exam findings given the region of the brain affected by the hemorrhage. Here’s a nice table detailing these changes on the left.
Diagnosis
It goes without saying, ICH is a medical emergency. Head CT is diagnostic as it tells us the size and location and can also tell us about extension into the ventricular system, associated edema, and herniation risk. MRI is equal to CT for detection of acute ICH, but MRI is better for chronic ICH and takes more time to acquire the images, and its availability has limitations.
Predicting hemorrhage expansion: Spot and Swirl Sign are regularly tested, high yield markers that suggest increased risk for hemorrhage expansion.
· Spot sign: small focal areas of contrast enhancement within a hemorrhage on CTA. It is linked to poorer outcomes and higher mortality. It looks like a bright white dot inside the hemorrhage.9
· Swirl Sign: rounded, linear, or irregular regions that are hypodense inside the hemorrhage.10
Labs: All patients should have a PT with INR, activated partial thromboplastin time (aPTT), and complete blood count (CBC) with platelet count. If taking LMWH, add on a factor Xa level if your hospital does it in-house. If on warfarin, an INR> 1.4 indicates they are anticoagulated. If they are on a factor Xa inhibitor, it is important to note the last time they took it. Anticoagulation can be based on a ingestion within a period of five half-lives and/or laboratory evidence of anticoagulant effect (eg, INR> 1.4 or an increased anti-factor Xa activity).
· Clinical Pearl: ICH while on warfarin usually occurs with a therapeutic level of anticoagulation (INR 2.0 to 3.5)
Management
See our handout on our website regarding the management of increased ICP. The two cornerstones of rapid therapy that must occur ASAP in the ED include reversal of anticoagulation and BP management.
Anticoagulation reversal: all anticoagulative agents should be discontinued, and their effects should be reversed.11
Warfarin reversal: 10 mg of IV vitamin K at 0.5-1 mg/min (takes 12-24 hrs to work). Given the delayed onset, you will also need a 4-factor prothrombin complex concentrate (PCC) at 50 units/kg or a fixed 2000 units. If 4-factor PCC is not available, you can use 3-factor PCC combined with rFVIIa or fresh frozen plasma (FFP).
*PCC has been found to have a faster onset, less volume administered, and fewer side effects as it is manufactured and not requiring type and screen. It is the go-to agent, preferred in most institutions. Thrombosis risk is low at 14-days, around 3%.
Heparin reversal: protamine sulfate is recommended. It can be given as a slow IV infusion. Its dose is dependent on the dose of heparin given.
Any patients with severe coagulation factor deficiency or thrombocytopenia should have this corrected immediately with an appropriate factor or platelet replacement.
What about the DOACs? These are difficult, and only a few have direct reversal agents.
Dabigatran reversal: Idarucizumab. If unavailable or there if continued bleeding, use 4 factor PCC.
Rivaroxaban, apixaban, edoxaban, fondaparinux, betrixaban reversal: For these Factor Xa inhibitors, Andexanet alfa or PCC are used. There is no definitive study to support any advantage of Andexanet alfa over PCC in favorable outcomes. The former is much more expensive and not available at many facilities.
BP management- this is a debated area, with the guidelines continually shifting. Let’s summarize the most basic, general recommendations.11,12
· For patients with SBP 160-220, lower to a target of ~140 SBP.
· Reducing below 140 SBP is not beneficial and should be avoided.
· For patients with SBP >220, lower to ~140-160 SBP.
· Preferred agents: clevidipine drip > nicardipine drip, labetalol, esmolol, or enalaprilat.
Prognosis
30-day mortality is not good, ranging from 30-50%. Perhaps more sobering, one-half of these patients die in the first 48 hours. Recurrence is 1-7%, higher in those with lobar ICH. Recurrence is most common within the first year of hemorrhage.13,14
References
1. Cordonnier C, Demchuk A, Ziai W, Anderson CS. Intracerebral haemorrhage: current approaches to acute management. Lancet 2018; 392:1257.
2. Beslow LA, Licht DJ, Smith SE, et al. Predictors of outcome in childhood intracerebral hemorrhage: a prospective consecutive cohort study. Stroke 2010; 41:313.
3. He J, Whelton PK, Vu B, Klag MJ. Aspirin and risk of hemorrhagic stroke: a meta-analysis of randomized controlled trials. JAMA 1998; 280:1930.
4. Thrift AG, McNeil JJ, Forbes A, Donnan GA. Risk of primary intracerebral haemorrhage associated with aspirin and non-steroidal anti-inflammatory drugs: case-control study. BMJ 1999; 318:759.
5. Xu BN, Yabuki A, Mishina H, et al. Pathophysiology of brain swelling after acute experimental brain compression and decompression. Neurosurgery 1993; 32:289.
6. Brott T, Broderick J, Kothari R, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 1997; 28:1.
7. Hanley DF. Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke 2009; 40:1533.
8. Al-Shahi Salman R, Frantzias J, Lee RJ, et al. Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. Lancet Neurol 2018; 17:885.
9. Wada R, Aviv RI, Fox AJ, et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke 2007; 38:1257.
10. Selariu E, Zia E, Brizzi M, Abul-Kasim K. Swirl sign in intracerebral haemorrhage: definition, prevalence, reliability and prognostic value. BMC Neurol 2012; 12:109.
11. Hemphill JC 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015; 46:2032.
12. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018; 138:e484.
13. Fogelholm R, Murros K, Rissanen A, Avikainen S. Long term survival after primary intracerebral haemorrhage: a retrospective population based study. J Neurol Neurosurg Psychiatry 2005; 76:1534.
14. Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2014; 85:660.



