Want to experience the greatest in board studying? Check out our interactive question bank podcast- the FIRST of its kind here: emrapidbombs.supercast.com
Author: Blake Briggs, MD
Peer Reviewer: Travis Smith, DO
Introduction
Cervical artery dissections are a common cause of stroke in young people <50 years old with some reports of up to 20% being from dissections. They can occur at any age. Much like aortic dissections, there is some loss of structure along the wall of either the internal carotid artery (ICA) or vertebral artery (VA), allowing blood to collect within the intima. This review will focus on spontaneous dissections, not traumatic, as well as the pathophysiology, risk factors, presentation, diagnosis, and management.
In patients <50 years old, cervical artery dissections account for 20% of ischemic strokes. The overall incidence is about 2.5 per 100,000 annually in the US alone, with a mean age of 45 years. ICA dissections are 3-5x more common that VA dissections. For comparison, subarachnoid hemorrhage (SAH), another can’t-miss neurological diagnosis, has an incidence of ~7 per 100,000 worldwide.
Pathophysiology
As stated above, a separation of the arterial wall layers results in dissection. They occur extracranial or intracranial. Extracranial internal carotid dissection is more common, typically it occurs 2 cm or more distal from the carotid bifurcation (near the skull base). Vertebral artery dissection most commonly occurs at the V3 segment of the vertebral artery, at the C1-C2 level.
The false lumen that forms will continue to dissect, or “unzip”, resulting in cerebral ischemia. Ischemia is caused by both hypoperfusion or thromboembolism, with the latter being more important. The compression of the expanding hematoma presses on sympathetic fibers on the outside of the blood vessel and adjacent nerves, resulting in partial Horner syndrome (see below), cranial neuropathies, and pain.
Very rarely these vessels dissect intracranially, rupturing to cause a SAH.
Accelerate your learning with our EM Question Bank Podcast
- Rapid learning
- Interactive questions and answers
- new episodes every week
- Become a valuable supporter
Risk Factors
Minor trauma: the key word is “mild” trauma, associated with up to 40% of cases. This does not include motor vehicle crashes that end up at a level 1 trauma center with a cervical spine fracture. These are usually activity-related: skating, basketball, volleyball, swimming, scuba diving, dancing, yoga, chiropractors (1 in 20,000 cervical manipulations), sexual intercourse, trampoline use, amusement park rides, vaginal delivery, etc.
With that being said, most CADs develop in the absence of any discernible mechanical event thus making it very difficult to indict a particular incident or factor as the cause of a particular stroke.
The most compelling risk factors likely have to do with an underlying connective tissue abnormality, which likely contribute to a weakening of the vascular wall. These genetic predispositions are the usual culprits (Ehlers-Danlos, Marfan’s, PCKD, etc). The most common of these is fibromuscular dysplasia.
While both vertebral and cervical dissections are associated with minor trauma, vertebral dissection has a higher rate and they are more associated with rapid head movements.
Presentation
Think headache, neck pain, and potentially ischemic, neurologic symptoms.
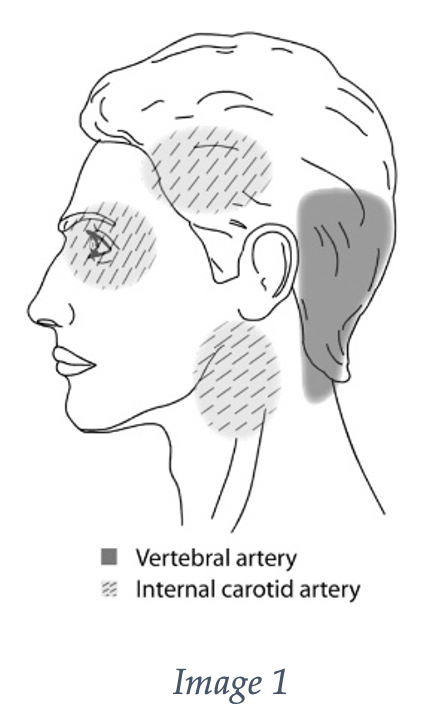
It is important to add this pathology to your differential of patients with headache and neck pain, as headache and neck pain are the most common symptoms, ranging from 60-90%. Headache is more common in carotid dissections, while neck pain is more common in vertebral dissection. See image 1 for the common associated pain locations.
The headache onset is usually gradual, with <20% being a “thunderclap” onset. Both vertebral and carotid dissections have no difference in terms of occurrence and frequency of headache as a presenting symptom, as well as thromboembolism or mural thrombus hematoma.
Ischemia manifesting in TIA/stroke symptoms are present in about 70% of patients but this may not be present on initial presentation. The risk is highest during the first 2 weeks of symptoms (77% present at the time of diagnosis).
On exam, look for the obvious motor or sensory deficits in addition to the more subtle nystagmus, truncal ataxia (can’t sit up in bed), ipsilateral Horner’s syndrome, tongue deviation, or an ophthalmoplegia.
For vertebral dissections think of lateral medullary syndromes (Wallenberg Syndrome) and cerebellar infarctions.
Think amaurosis for carotid artery dissection.
As mentioned above, ipsilateral Horner syndrome is only in ~25% of cases. Even so, it is usually partial, with only unilateral ptosis and miosis. Anhidrosis is rarely seen.
Other possible symptoms include unilateral hearing loss, pulsatile tinnitus, auscultated bruit, dizziness, the “Deadly D’s”(dysarthria, diplopia, dysphagia), other cranial neuropathies, and scalp tenderness.
Diagnosis
Made by neuroimaging, MRA or CTA. Both are more or less equal in performance and sensitivity/specificity. CTA is faster and has wider availability in most EDs. Historically, the gold standard was digital subtraction angiography, but this is rarely used today.
Key findings on CT imaging: vessel stenosis, tapered occlusion, dissecting aneurysm (pseudoaneurysm), intimal flap, double lumen, or intramural hematoma.
Rarely, CTA and MRA can be falsely negative. If you have a strong suspicion for cervical artery dissection, proceed with arranging digital subtraction angiography.
Ultrasound can be used but its limitations include intracranial extension and imaging of the vertebral artery system. Additionally, the fact that it has been found to show evidence specific to dissection in only 20% of patients makes it an imaging modality to forego.
Treatment
As discussed above, cervical artery dissection increases the risk of thromboembolism, causing stroke. This can be a deadly diagnosis as there is up to a 10% mortality prior to even initiating treatment.
In those patients who present with acute ischemic stroke, standard approaches to management of stroke should be followed. Discussion of thrombolytics should take place with the code stroke team.
There is no increased harm in giving IV thrombolytics (outside of the usual harm associated with acute stroke management), but no clear benefit has been found as well (i.e. no difference in intracranial hemorrhage, mortality, or favorable outcome).
One critical caveat is that thrombolytics are relatively contraindicated in those with intracranial extension of the dissection. Another complication that is quite obvious: a cervical artery dissection involving the aorta…we’ll let you think about why that would be bad.
Anti-thrombotic therapy with either antiplatelet agents or anticoagulation are the preferred methods for preventing ischemic stroke and TIA complications. They have been studied and there is no difference in their efficacy.
There is debate on the preferred antiplatelet agents: aspirin, clopidogrel, dipyridamole, or a combination of these agents. We suggest consulting your neurologic team on this.
There is no consensus on how long patients need to be on therapy. Some studies suggest after 3-6 months as most arterial abnormalities stabilize by then. Repeat imaging is necessary at that time to evaluate for improvement in the dissection complications.
Endovascular or surgical repair are last resort options in those who have recurrent ischemia or failed antithrombotic therapy. The subject of endovascular surgery is institutionally dependent, along with who runs the service and CTA findings.
Prognosis
Recurrence rate is uncertain, and hotly debated. We do know that unfavorable outcomes are more common in carotid artery dissections versus vertebral. Excellent recovery has been found in 70-85% of patients, with major disabling deficits in 10-25% and death in <10%.
References
- Pannone A, Lucchetti G, Stazi G, et al. Internal carotid artery dissection in a patient with Behçet’s syndrome. Ann Vasc Surg 1998; 12:463.
- Völker W, Dittrich R, Grewe S, et al. The outer arterial wall layers are primarily affected in spontaneous cervical artery dissection. Neurology 2011; 76:1463.
- Lucas C, Moulin T, Deplanque D, et al. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke 1998; 29:2646.
- Morel A, Naggara O, Touzé E, et al. Mechanism of ischemic infarct in spontaneous cervical artery dissection. Stroke 2012; 43:1354.
- Mattioni A, Paciaroni M, Sarchielli P, et al. Multiple cranial nerve palsies in a patient with internal carotid artery dissection. Eur Neurol 2007; 58:125.
- Downer J, Nadarajah M, Briggs E, et al. The location of origin of spontaneous extracranial internal carotid artery dissection is adjacent to the skull base. J Med Imaging Radiat Oncol 2014; 58:408.
- Lee VH, Brown RD Jr, Mandrekar JN, Mokri B. Incidence and outcome of cervical artery dissection: a population-based study. Neurology 2006; 67:1809.
- Engelter ST, Grond-Ginsbach C, Metso TM, et al. Cervical artery dissection: trauma and other potential mechanical trigger events. Neurology 2013; 80:1950.
- Karnik R, Rothmund T, Bonner G, et al. Inline skating as a possible cause of consecutive bilateral vertebral artery dissection. Acta Neurol Scand 2000; 101:70.
- Abe A, Nishiyama Y, Kamiyama H, et al. Symptomatic middle cerebral artery dissection in a young tennis player. J Nippon Med Sch 2009; 76:209.
- De Giorgio F, Vetrugno G, De Mercurio D, et al. 1. Dissection of the vertebral artery during a basketball game: a case report. Med Sci Law 2004; 44:80.
- Slankamenac P, Jesic A, Avramov P, et al. Multiple cervical artery dissection in a volleyball player. Arch Neurol 2010; 67:1024.
- Furtner M, Werner P, Felber S, Schmidauer C. Bilateral carotid artery dissection caused by springboard diving. Clin J Sport Med 2006; 16:76.
- Bartsch T, Palaschewski M, Thilo B, et al. Internal carotid artery dissection and stroke after SCUBA diving: a case report and review of the literature. J Neurol 2009; 256:1916.
- Faivre A, Abdelfettah Z, Rodriguez S, Nicoli F. Neurological picture. Bilateral internal carotid artery dissection due to elongated styloid processes and shaking dancing. J Neurol Neurosurg Psychiatry 2009; 80:1154.
- Caso V, Paciaroni M, Bogousslavsky J. Environmental factors and cervical artery dissection. Front Neurol Neurosci 2005; 20:44.
- Wechsler B, Kim H, Hunter J. Trampolines, children, and strokes. Am J Phys Med Rehabil 2001; 80:608.
- Burneo JG, Shatz R, Papamitsakis NI, Mitsias PD. Neuroimages: amusement park stroke. Neurology 2000; 55:564.
- Faivre A, Chapon F, Combaz X, Nicoli F. Internal carotid artery dissection occurring during intensive practice with Wii video sports games. Neurology 2009; 73:1242.
- Delasobera BE, Osborn SR, Davis JE. Thunderclap headache with orgasm: a case of basilar artery dissection associated with sexual intercourse. J Emerg Med 2012; 43:e43.
- Paciaroni M, Bogousslavsky J. Cerebrovascular complications of neck manipulation. Eur Neurol 2009; 61:112.
- Olin JW, Gornik HL, Bacharach JM, et al. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation 2014; 129:1048.
- Kim BM, Kim SH, Kim DI, et al. Outcomes and prognostic factors of intracranial unruptured vertebrobasilar artery dissection. Neurology 2011; 76:1735.
- Debette S, Grond-Ginsbach C, Bodenant M, et al. Differential features of carotid and vertebral artery dissections: the CADISP study. Neurology 2011; 77:1174.
- Mitsias P, Ramadan NM. Headache in ischemic cerebrovascular disease. Part I: Clinical features. Cephalalgia 1992; 12:269.
- Pelkonen O, Tikkakoski T, Luotonen J, Sotaniemi K. Pulsatile tinnitus as a symptom of cervicocephalic arterial dissection. J Laryngol Otol 2004; 118:193.
- Provenzale JM, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol 2009; 193:1167.
- Kasner SE. CADISS: a feasibility trial that answered its question. Lancet Neurol 2015; 14:342.
- Engelter ST, Rutgers MP, Hatz F, et al. Intravenous thrombolysis in stroke attributable to cervical artery dissection. Stroke 2009; 40:3772.
- CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol 2015; 14:361.
- Markus HS, Levi C, King A, et al. Antiplatelet Therapy vs Anticoagulation Therapy in Cervical Artery Dissection: The Cervical Artery Dissection in Stroke Study (CADISS) Randomized Clinical Trial Final Results. JAMA Neurol 2019; 76:657.
- Kennedy F, Lanfranconi S, Hicks C, et al. Antiplatelets vs anticoagulation for dissection: CADISS nonrandomized arm and meta-analysis. Neurology 2012; 79:686.
- Nedeltchev K, Bickel S, Arnold M, et al. R2-recanalization of spontaneous carotid artery dissection. Stroke 2009; 40:499.
- Donas KP, Mayer D, Guber I, et al. Endovascular repair of extracranial carotid artery dissection: current status and level of evidence. J Vasc Interv Radiol 2008; 19:1693.
- Arnold M, Bousser MG, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke 2006; 37:2499.
- Haneline MT, Rosner AL. The etiology of cervical artery dissection. J Chiropr Med. 2007;6(3):110-120. doi:10.1016/j.jcme.2007.04.007
- Britt TB, Agarwal S. Vertebral Artery Dissection. [Updated 2020 Jul 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441827/



