Want to experience the greatest in board studying? Check out our interactive question bank podcast- the FIRST of its kind here: emrapidbombs.supercast.com
Author: Blake Briggs, MD
Peer Reviewer: Iltifat Husain, MD
Introduction
Nothing strikes more fear and suspense in healthcare than when discussing “abdominal aortic aneurysms” (AAA). Perhaps it is due to their utterly silent growth and potential for catastrophic rupture. AAA is the most common “true” aneurysm in the human body.1 In developed countries, the prevalence is estimated between 2-8%, with men nearly twice as affected.2 From screening, it is estimated nearly 1,000,000 people in the US alone have a AAA.3 Since the 1990’s, mortality from AAA has dramatically decreased by ~50%, likely secondary to rates of smoking cessation, more awareness and screening, and improved vascular surgery techniques. It continues to remain a very difficult diagnosis due to its ability to remain virtually silent until rupture. This review will focus on the risk factors, screening definitions, presentation and diagnosis of a patient with a AAA. The techniques of AAA bedside ultrasound are outside the scope of this review.
Anatomy and definitions
The mighty abdominal aorta is a retroperitoneal structure. It begins at the diaphragm and extends to its bifurcation into the right and left common iliac arteries in the lower abdomen (usually below the umbilicus, L4 level).
In adults, >3.0 cm diameter at any location along the abdominal aorta is an aneurysm.
The most common location for a AAA is the segment between the renal and inferior mesenteric arteries.4
Small aneurysms <4.0 cm
Accelerate your learning with our EM Question Bank Podcast
- Rapid learning
- Interactive questions and answers
- new episodes every week
- Become a valuable supporter
Medium aneurysms 4.0-5.5 cm
Large >5.5 cm
Risk Factors
The most important factor for aneurysm expansion is ongoing smoking.5
Other risks include: older age, Caucasian race, hypertension, family history of AAA, presence of other aneurysms in the body, and atherosclerosis (#obviously).2
“The most important risk factor for rupture is aneurysmal size.”
AAA’s will naturally expand. The rate is variable and depends on patient factors, most importantly being size. Let’s repeat that again: size is the most important risk factor in determining AAA expansion and rupture. Any aneurysm >5.5 cm is at the highest risk of rupture. Trust us, that is an easy test question you don’t want to miss.
Other risks for rupture include fast rate of expansion (>0.5 cm over a 6-month period), those who smoke, females, and those with poorly controlled hypertension.
On average, AAA’s expand at a rate of 0.3-0.4 cm per year.6 Some aneurysms could remain stable for years then undergo rapid expansion (#scary).
Screening
Screening has been shown to reduce AAA-related mortality in men >65 years old. Otherwise, in those with a low risk for AAA, the benefit is likely very small.
Who you really need to think about AAA in:
· Any male 65-75 who has ever smoked
· Any male or female 65-75 who has a first-degree relative with a AAA
Presentation
The majority of AAA patients are asymptomatic. The history and exam are essentially no help, and therefore it is up to the clinician to think about AAA when a patient presents with risk factors.7
Only about 30% of patients will have a palpable, pulsatile abdominal mass. The ability to find these really depends on clinician experience, patient body habitus, and aneurysm size. Of note, palpating a AAA has never been shown to precipitate rupture.8
~5-22% of patients with AAA have attributable symptoms.9 Symptoms of a AAA include abdominal pain, flank pain, or back pain. Pelvic and groin pain are also classically described. Overall, pain is found in 75% of those with symptoms. Pre-syncope or syncope is seen in 30%.10 Large AAAs might have a bruit that can be auscultated, but this is unreliable.
Rarely, limb ischemia has been associated with AAA, as embolus or thrombus from atherosclerotic plaques from the aneurysm break off and travel to the distal extremities.11
Rarely, fever, chronic malaise, or weight loss may be present, suggesting an inflammatory aneurysm, often associated with infection.12
“It behooves you to always think about AAA in any patient age >65 who presents to the ED with abdominal pain, back pain, or flank pain. Taking that extra 5 minutes to think about AAA is always the right thing to do. ”
Look at their prior records: prior CT’s with the past few years should show a AAA if present. A high percentage of patients may not realize they have a AAA (20-30%), and others may not understand what you are asking about or if their symptoms are attributable.
You can always just do a quick bedside ultrasound of their abdomen if you are not planning on CT, and this is a pro-move we encourage at our shop. We encourage measuring three transverse views (proximal, middle, distal), followed by 2 longitudinal views. Make sure you capture the point of bifurcation of the aorta to the iliac arteries. Always measure the diameter from the outside wall, as in the picture to the right we caught on bedside ultrasound. One would be initially fooled that the true lumen is only the inner circle (orange arrow), but in reality, it should be measured from the dotted line.
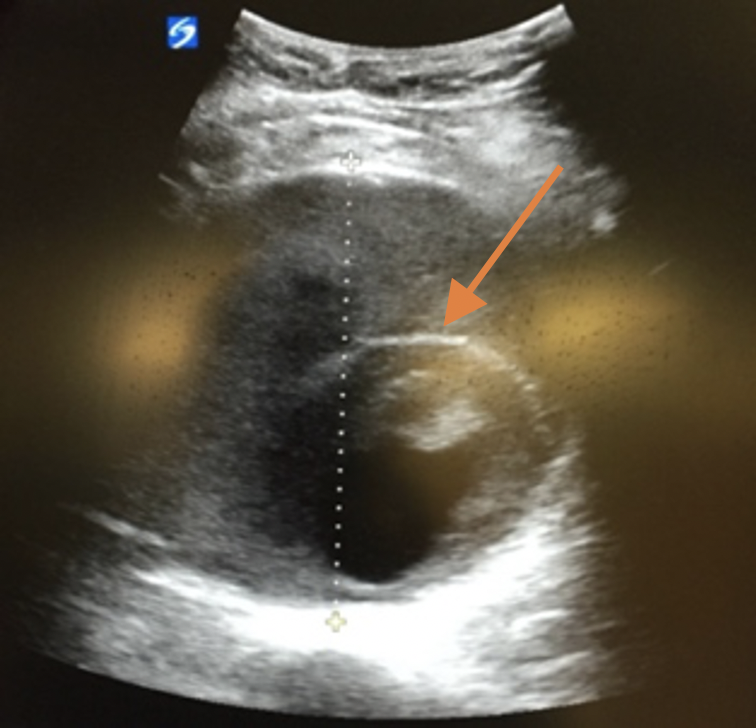
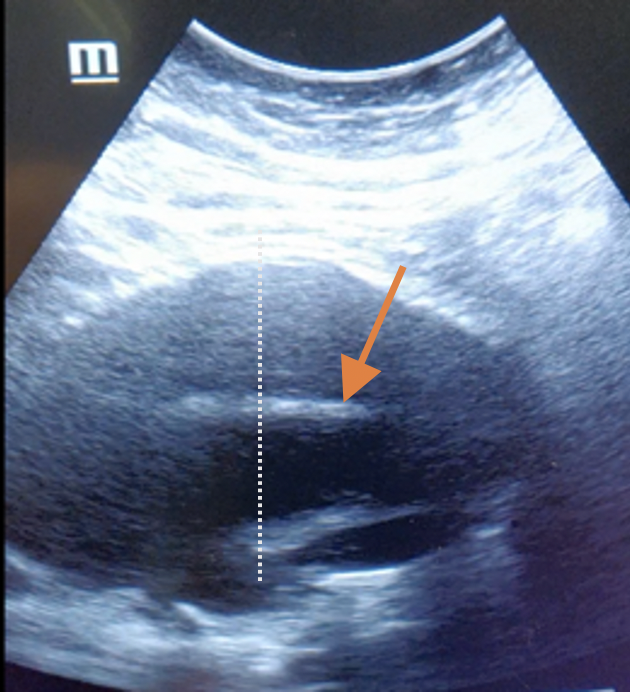
Ruptured AAA
Only 20-30% of patients who present to the ED with a AAA rupture have a known history of AAA.4 The classic triad of severe pain, hypotension, and a pulsatile abdominal mass only occur in 50% of patients.13 The classic teaching is that these patients immediately “develop shock and die”. This is partially true. 50% of patients with rupture survive long enough for treatment, and those that do may have nonspecific symptoms and may even delay medical attention.
Even worse, in those presenting with symptoms, misdiagnosis is common. ~30% of patients will have their symptoms misdiagnosed as renal colic, perforated viscous, diverticulitis, GI bleeding, or ischemic bowel.
The AAA most commonly ruptures into the retroperitoneum but can bleed into the peritoneum as well. Rarely, Gray-Turner sign (flank ecchymosis) or Cullen’s Sign (periumbilical ecchymosis), may be seen, but they are not specific to the diagnosis of AAA.
Diagnosis
The steps for AAA diagnosis depend on the patient’s presentation (see algorithm below).
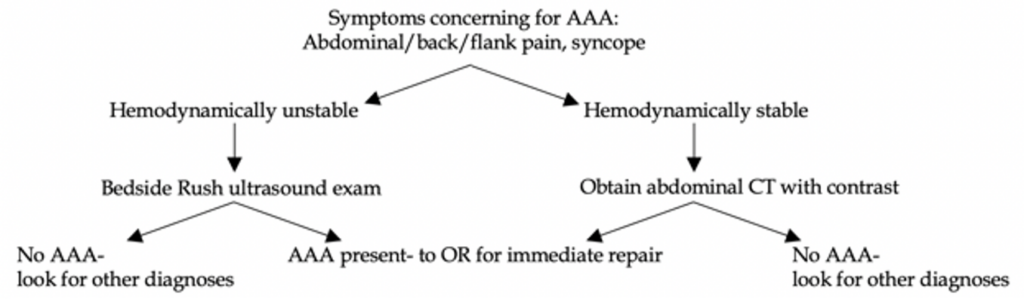
In hemodynamically unstable patients who present to the ED, a “RUSH exam” (Rapid Ultrasound for Shock and Hypotension) is a great start as you resuscitate your patient. Looking at the abdomen via the eFAST views (each lung, RUQ, LUQ, suprapubic, subxiphoid) and a quick, sliding view of the abdominal aorta is an excellent screening tool.14
As one would expect, labs are not helpful. If the patient has a ruptured AAA, their labs may reflect end-organ damage, but this is nonspecific and can lead you down the wrong diagnostic path as well if you are not expecting a AAA.
For those asymptomatic: abdominal vascular ultrasound is preferred, with sensitivity and specificity approaching 100%.15 It has no radiation and is noninvasive.
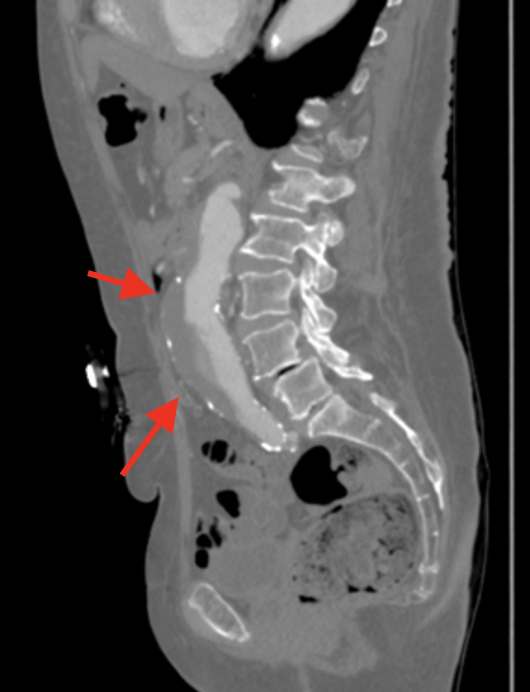
For those symptomatic who are hemodynamically stable, but with potential for other causes of abdominal pain, back pain, or flank pain, abdominal CT with contrast is the test of choice. CT can show obvious signs of rupture including retroperitoneal hematoma, and contrast extravasation. See pic to to the right.
CT might also detect “unstable” signs of impending rupture such as a crescent sign of layering hematoma in the aorta, breaks in the aortic wall calcification, and aortic blebs that emerge from the aortic surface.16
Management
Those with ruptured AAA require emergent surgery for hemorrhage control and repair. Simple as that. Acting fast can save the patient’s life. Your duties while the patient is in the ED consists of the following:
1. Aggressive management with large bore IVs (14-16g). Ultrasound-guidance might be needed in these patients due to their blood loss status, and you can be a ninja placing them.
2. Emergent release blood in whom rupture is suspected. Skip the crystalloids.
3. If your RUSH exam detects free fluid in the abdomen, immediately call surgery.
If resuscitation is prompt and aggressive, and the patient improves clinically enough for CT to be performed prior to the OR, this is fine but never delay calling vascular surgery in a hemodynamically unstable patient with a AAA.
Any patients with symptoms that could be attributed to their AAA should be admitted to the hospital and vascular surgery consulted in the ED. Remember, if you admit any patient to the hospital secondary to their AAA, ruptured or not, it counts as critical care billing!
Regardless of size, aneurysm repair is indicated for patients with symptoms (abdominal pain/flank pain/back pain) that cannot be attributed to another etiology.
Asymptomatic patients who are discovered incidentally to have a AAA in the ED require close outpatient follow up. Their follow up will consist of repeat US screening of the aneurysm and potentially a vascular surgery consult in the office setting. Their discussion regarding repair really comes down to if the risk of rupture exceeds the risk of repair and vice versa.
Unfortunately, up to ~30% of patients with incidental aneurysms found on imaging did not undergo subsequent monitoring. It is our duty in the ED, especially in the era of “pan-scans”, to inform patients of their findings.17
References:
1. Johnston KW, Rutherford RB, Tilson MD, et al. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg 1991; 13:452.
2. Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg 2010; 52:539.
3. Mussa FF. Screening for abdominal aortic aneurysm. J Vasc Surg 2015; 62:774.
4. Sandhu RS, Pipinos II. Isolated iliac artery aneurysms. Semin Vasc Surg 2005; 18:209.
5. Sweeting MJ, Thompson SG, Brown LC, et al. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg 2012; 99:655.
6. Gadowski GR, Pilcher DB, Ricci MA. Abdominal aortic aneurysm expansion rate: effect of size and beta-adrenergic blockade. J Vasc Surg 1994; 19:727.
7. Fink HA, Lederle FA, Roth CS, et al. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med 2000; 160:833.
8. Lederle FA, Simel DL. The rational clinical examination. Does this patient have abdominal aortic aneurysm? . JAMA 1999; 281:77.
9. Sullivan CA, Rohrer MJ, Cutler BS. Clinical management of the symptomatic but unruptured abdominal aortic aneurysm. J Vasc Surg 1990; 11:799.
10. Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromsø Study, 1994-2001. Circulation 2009; 119:2202.
11. Hirose H, Takagi M, Hashiyada H, et al. Acute occlusion of an abdominal aortic aneurysm–case report and review of the literature. Angiology 2000; 51:515.
12. Pennell RC, Hollier LH, Lie JT, et al. Inflammatory abdominal aortic aneurysms: a thirty-year review. J Vasc Surg 1985; 2:859.
13. Rinckenbach S, Albertini JN, Thaveau F, et al. Prehospital treatment of infrarenal ruptured abdominal aortic aneurysms: a multicentric analysis. Ann Vasc Surg 2010; 24:308.
14. Lee SI, Mueller PR, Thrall JH. Re: “managing incidental findings on abdominal CT: White Paper of the ACR Incidental Findings Committee”. J Am Coll Radiol 2011; 8:e3.
15. LaRoy LL, Cormier PJ, Matalon TA, et al. Imaging of abdominal aortic aneurysms. AJR Am J Roentgenol 1989; 152:785.
16. Boules TN, Compton CN, Stanziale SF, et al. Can computed tomography scan findings predict “impending” aneurysm rupture? Vasc Endovascular Surg 2006; 40:41.
17. Lederle FA, Johnson GR, Wilson SE, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med 1997; 126:441.


