Want to experience the greatest in board studying? Check out our interactive question bank podcast- the FIRST of its kind here: emrapidbombs.supercast.com
Author: Blake Briggs
Peer Reviewers: Travis Smith, DO, Sean O’Sullivan, MD
Introduction
Intestinal ischemia is any process that causes reduced blood flow to the small or large bowel. They are a collection of various syndromes that are; all often difficult to diagnose but can lead to catastrophic consequences. Overall, the incidence has been increasing, likely due to an elderly population in the US as well as higher clinician awareness.
Definitions: Let’s set the record straight. There are different types of intestinal ischemia, all are bad.
o Mesenteric Ischemia = small bowel
o Colonic Ischemia = large bowel
o Splanchnic ischemia = intestines plus kidney, liver, or spleen
o Chronic mesenteric ischemia: develops in atherosclerotic patients causing episodic hypoperfusion related to eating.
Accelerate your learning with our EM Question Bank Podcast
- Rapid learning
- Interactive questions and answers
- new episodes every week
- Become a valuable supporter
Acute Mesenteric Ischemia: sudden small intestinal hypoperfusion due to mesenteric arterial embolism (50 percent), mesenteric arterial thrombosis (15 to 25 percent), mesenteric venous thrombosis (5 percent), and non-occlusive mesenteric ischemia due to intestinal hypoperfusion (20 to 30 percent). The true rates are not exactly known with some studies showing a prevalence of 1 out of 1000 hospital admissions. One prospective study in Sweden showed about ~5 per 100,000 per year (…but that’s Sweden).
Brief Relevant Anatomy
Arterial supply to the intestines is made up of the SMA and IMA. The celiac artery provides some collateral blood flow, mainly to the duodenum. The SMA basically supplies the entire small intestine except the proximal duodenum. The SMA and IMA supply the colon. We will not cover the collateral blood supply network (surgeons dream about this).
The SMA is overwhelming the most commonly affected vessel due to its large diameter and narrow takeoff angle.
Danger Zone area
According to Kenny Loggins, there are two major watershed areas prone to ischemia, otherwise known as “danger zones”. The classic, most commonly tested one is the splenic flexure, supplied by some weak-ass, little branches of the SMA.
The second is the rectosigmoid junction, supplied by some measly vessels from the IMA.
Pathophysiology of intestinal ischemia
Remember that the splanchnic circulation is at the mercy of systemic vascular resistance. Normally it receives 10-35% of cardiac output, but this widely fluctuates based on stress, and the fed or fasted state. Intestinal oxygenation extraction is also low, allowing remaining oxygen to be delivered to the liver via the portal vein. The sympathetic nervous system in times of stress (infection, inflammation, shock), will vasoconstrict the majority of splanchnic circulation, thus allowing blood flow to more “vital organs” (e.g. brain).
Intestinal blood flow only has to be reduced by 50% from normal fasting level baseline before ischemia occurs. Couple this with atherosclerosis and/or thromboembolism in high risk patients and you have a dangerous setup.
Risk Factors
Look for vascular patients. Anyone with serious atherosclerotic burden (advanced age, coronary artery disease, stroke, peripheral arterial disease, prior aortic surgery, polyarteritis nodosa). Also, any patient that has pathophysiology for throwing clots from the heart: endocarditis, cardiac arrhythmias (e.g. A fib), ventricular aneurysm.
Presentation
The history is the most important piece of the puzzle. The physical exam sucks, the labs suck, and a CT angiogram of the abdomen/pelvis without oral contrast is great but not that great. So you need to start with a high index of suspicion. Very high. The most common presenting complaint is sudden abdominal pain.
Classically, “abdominal pain out of proportion to physical exam”. Easier said than done. This is only seen in ~40% of patients. Patients vary in their pain thresholds, and despite this old adage usually being true, inexperienced providers will often miss this condition. Not to mention the majority of these patients are >65 years old. This is perhaps why mesenteric ischemia is so difficult to diagnose.
Why pain out of proportion? These are visceral pain fibers from the splanchnic circulation. Remember that the visceral innervation is not as specific and regionally correlative as parietal innervation.
Blood in stool (often hematochezia), is concerning and should really raise suspicion. However, it is unreliable- present in less than <50% of patients.
Nonspecific symptoms like nausea and vomiting are common.
Personal and family history is very important, remember to ask about the above risk factors
A history of prior embolization is a major red flag, but only found in 1/3 of patients. In one autopsy series, 20% previously had an acute MI, 50% had cardiac thrombi.
Pain after eating should be a red flag warning, as this is due to an inability to increase blood flow to meet demand (most common in chronic mesenteric ischemia).
Physical exam is unhelpful here. No focal tenderness, and often it can be normal. Occult blood may be found in the stool which can raise suspicion. A complete vascular exam should be performed, measuring carotid, radial, femoral, and DP pulses for synchronous embolism. Over 20% of acute mesenteric ischemia cases are multiple emboli!
Laboratory studies stink. In a minority of patients, there can be nonspecific leukocytosis or lactic acid elevation, even less common, metabolic acidosis. All are late findings.
Everyone talks about lactate elevation, and its sensitivity is 86% for acute mesenteric ischemia (specificity 44%). However, lactates will not be elevated until late disease, so a normal lactate cannot reliably exclude the diagnosis.
Normal D-dimer levels may help to exclude acute intestinal ischemia as a study examining found that no patient presenting with AME had an elevated level. But just like its use in PE/DVT, an elevated level is not specific. Also, use of d-dimer has not been validated.
Imaging
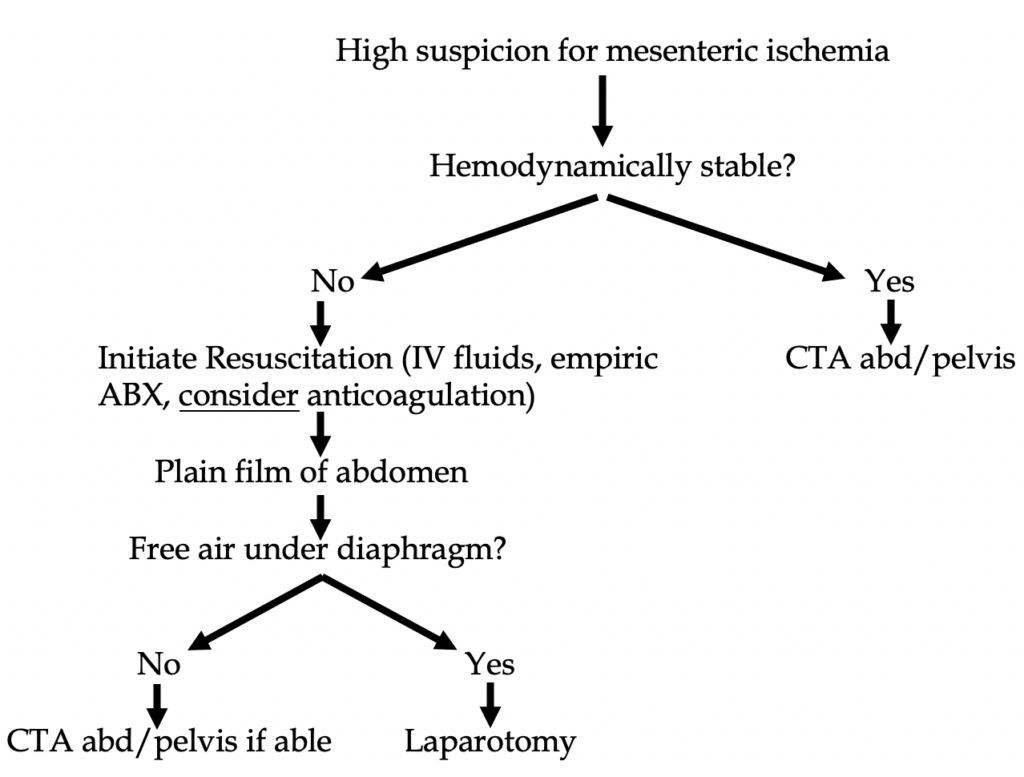
Plain films are virtually nondiagnostic for mesenteric ischemia, but in hemodynamically unstable patients they should be utilized for evaluation for free air, thus making obvious the need for laparotomy (see Figure 1 below).
CT angiogram (CTA) of the abdomen and pelvis with IV contrast: this is the best test in the ED. No oral contrast should be given as it will obscure the vessels. It is important to specify an angiogram vs regular CT abd/pelvis with IV contrast as the order dictates the timing of the contrast bolus, 20 secs for the angiogram vs 70 secs for the routine CT with contrast (portal venous phase). The CTA has a sensitivity of 93% and a specificity 96%.
Findings specific for mesenteric ischemia: focal or segmental bowel thickening, intestinal pneumatosis, bowel dilation, mesenteric stranding, organ infarction.
If the CTA is negative but suspicion remains high, a mesenteric arteriogram should be performed.
MR angiography: nearly equal to CT, possibly more sensitive but this does not pan out as it is more costly and slower. Good for patients with an allergy to iodinated contrast. (True allergies, not “I think I have an itching allergy to shellfish or Iodine”. Remember it’s impossible to be truly allergic to iodine).
Management
First step is to call a surgeon as those with peritoneal signs get an ex-lap with open surgical embolectomy. Second step includes fluid resuscitation, hemodynamic monitoring and support, correct electrolytes, pain control, antibiotics, and usually anticoagulation.
Importantly, avoid any vasoconstrictive agents. We prefer large volume of IV fluids in these cases, as IV fluids will allow for more splanchnic flow.
If vasopressors are used, low dose milrinone or inotropic agents like dobutamine or dopamine are preferred as they have less effects on mesenteric perfusion.
Broad spectrum coverage is recommended with Piperacillin/tazobactam being a preferred option for antibiotic coverage. Bowel infarction and ischemia can promote bacterial transmigration and if there are any microperforations present this can increase risk of peritonitis.
Anticoagulation: The mainstay is unfractionated heparin to limit thrombus propagation. A less established approach is with a local infusion of a thrombolytic agent; however, this option is reserved for patients with a shorter duration of symptoms and without signs of peritonitis.
Prognosis
Survival for acute ischemia is worse for arterial etiology rather than venous. These patients are very ill after surgery and often they stay in the hospital for a month with poor prognosis. Mortality rates exceed 60%! In one systemic review, the pooled operative mortality rate was 47%. Patients overall are still likely to die from the underlying condition that predisposed them to mesenteric ischemia.
References
1. Geoffrey D. Rubin (2012). CT and MR Angiography: Comprehensive Vascular Assessment. Lippincott Williams & Wilkins. p. 318. ISBN 9781469801834. Archived from the original on 2017-09-08.
2. Walker TG. Mesenteric vasculature and collateral pathways. Semin Intervent Radiol 2009; 26:167.
3. Meyers MA. Griffiths’ point: critical anastomosis at the splenic flexure. Significance in ischemia of the colon. AJR Am J Roentgenol 1976; 126:77.
4. Bulkley GB, Kvietys PR, Parks DA, et al. Relationship of blood flow and oxygen consumption to ischemic injury in the canine small intestine. Gastroenterology 1985; 89:852
5. Reinus JF, Brandt LJ, Boley SJ. Ischemic diseases of the bowel. Gastroenterol Clin North Am 1990; 19:319.
6. Cappell MS. Intestinal (mesenteric) vasculopathy. I. Acute superior mesenteric arteriopathy and venopathy. Gastroenterol Clin North Am 1998; 27:783.
7. Harward TR, Green D, Bergan JJ, et al. Mesenteric venous thrombosis. J Vasc Surg 1989; 9:328.
8. Glenister KM, Corke CF. Infarcted intestine: a diagnostic void. ANZ J Surg 2004; 74:260.
9. Cudnik MT, Darbha S, Jones J, et al. The diagnosis of acute mesenteric ischemia: A systematic review and meta-analysis. Acad Emerg Med 2013; 20:1087.
10. Li KC. Magnetic resonance angiography of the visceral arteries: techniques and current applications. Endoscopy 1997; 29:496.
11. Laissy JP, Trillaud H, Douek P. MR angiography: noninvasive vascular imaging of the abdomen. Abdom Imaging 2002; 27:488.
12. Hagspiel KD, Leung DA, Angle JF, et al. MR angiography of the mesenteric vasculature. Radiol Clin North Am 2002; 40:867.
13. Bradbury MS, Kavanagh PV, Chen MY, et al. Noninvasive assessment of portomesenteric venous thrombosis: current concepts and imaging strategies. J Comput Assist Tomogr 2002; 26:392.
14. Menke J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology 2010; 256:93.
15. Bala M, Kashuk J, Moore EE, et al. Acute mesenteric ischemia: guidelines of the World Society of Emergency Surgery. World J Emerg Surg 2017; 12:38.
16. Acosta S, Ogren M, Sternby NH, et al. Clinical implications for the management of acute thromboembolic occlusion of the superior mesenteric artery. Ann Surg 2005; 241:516.
17. Baddour LM, Wilson WR, Bayer AS, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015; 132:1435.
18. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005; 111:e394.
19. Cappell MS. Intestinal (mesenteric) vasculopathy. I. Acute superior mesenteric arteriopathy and venopathy. Gastroenterol Clin North Am 1998; 27:783.
20. Pagnoux C, Mahr A, Cohen P, Guillevin L. Presentation and outcome of gastrointestinal involvement in systemic necrotizing vasculitides: analysis of 62 patients with polyarteritis nodosa, microscopic polyangiitis, Wegener granulomatosis, Churg-Strauss syndrome, or rheumatoid arthritis-associated vasculitis. Medicine (Baltimore) 2005; 84:115.
21. Yamada T, Yoshii T, Yoshimura H, et al. Upper limb amputation due to a brachial arterial embolism associated with a superior mesenteric arterial embolism: a case report. BMC Res Notes 2012; 5:372.
22. Kougias P, Lau D, El Sayed HF, et al. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. J Vasc Surg 2007; 46:467.
23. Klempnauer J, Grothues F, Bektas H, Pichlmayr R. Long-term results after surgery for acute mesenteric ischemia. Surgery 1997; 121:239.
24. Corcos O, Castier Y, Sibert A, et al. Effects of a multimodal management strategy for acute mesenteric ischemia on survival and intestinal failure. Clin Gastroenterol Hepatol 2013; 11:158.
25. McKinsey JF, Gewertz BL . Acute mesenteric ischemia. Surg Clin North Am. 1997;77(2):307.
26. Block T, Nilsson TK, Björck M, Acosta S. Diagnostic accuracy of plasma biomarkers for intestinal ischaemia. Scand J Clin Lab Invest. 2008;68(3):242.

