Want to experience the greatest in board studying? Check out our interactive question bank podcast- the FIRST of its kind here: emrapidbombs.supercast.com
Author: Blake Briggs, MD
Peer Reviewer: Iltifat Husain, MD
“He’s more machine than man now” -Obi-Wan Kenobi when talking about LVADs.
Introduction
Since the beginning of modern medicine, management of end-stage congestive heart failure was extremely limited. With the recent advancements of mechanical assist devices, these have allowed a good bridge to transplant, recovery, or serve as a long-term “solution” by themselves. The one-year survival has improved steadily since 2001 (~50%; >90% in 2013). We do not have the exact population of total LVAD users in the US, but it’s been steadily increasing since the 2000s. Abbott claims they have had >26,000 patients implanted with their LVAD model, the HeartMate IITM. Unfortunately, the increase in LVAD presence throughout the US has increased the incidence of their associated complications. Understandably, a great number of EM physicians do not feel comfortable managing these patients- but you need to be! This document will serve as a brief review of what you need to know when it comes to LVADs and their complications.
Indications for LVAD placement
LVADs can be used in patients with end-stage congestive heart failure. It is often utilized as a bridge to heart transplant and as a therapy. It can only be used if the patient has a functioning right ventricle.
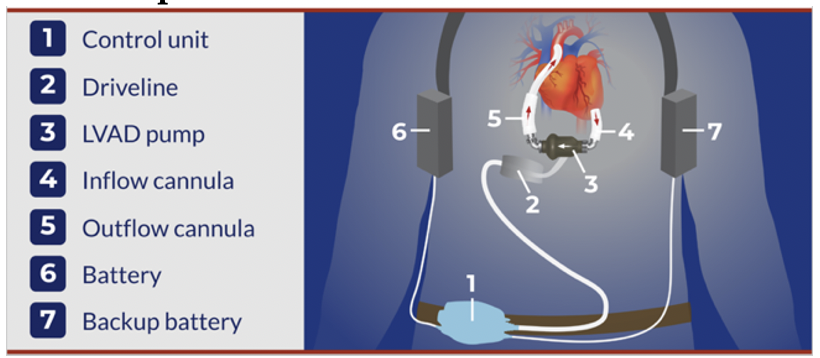
Basic Setup
LVADs are complex machines, but the premise is simple. The inflow cannula draws blood in from the LV to the pump, which then pumps it into the aorta via the outflow cannula. The system is either battery or wall powered.
Accelerate your learning with our EM Question Bank Podcast
- Rapid learning
- Interactive questions and answers
- new episodes every week
- Become a valuable supporter
If device sounds present but patient not breathing? Rescue breaths, intubate.
Most LVADs are designed as a “continuous flow” model, in which they lack pulsatile flow. Therefore, on exam your patient may not have a palpable pulse and might not have any discernible heart sounds. This also means measuring blood pressure can be difficult and misleading. In fact, automated BP cuffs are lame and only about 50% accurate. Pulse oximetry is also unreliable and likely reflects a lack of pulsatile flow.
How to measure BP in an LVAD patient: measure at the brachial artery, using a Doppler probe or ultrasound and a manual cuff, not automatic. Doppler is 95% accurate in LVAD patients, it is the best noninvasive measure. An acceptable MAP is 60-90 mmHg. The MAP should not exceed 90 mmHg, as this can compromise LVAD function.
What about a really ill-appearing LVAD patient and Doppler is unattainable? Place an arterial line. This is arguably the gold standard.
Basic facts you must know
The most common complication related to LVADs is bleeding. All LVAD patients are on anticoagulation. Invariably, bleeding is a common ED complaint.
The second most common complications of LVAD is infection.
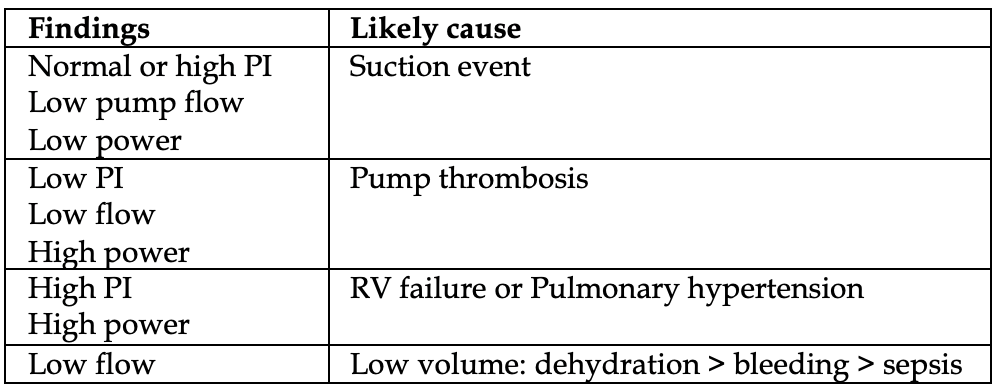
The most common cause of LVAD failure is a suction event.
The most common location for infection is the driveline. Remember that the driveline is the part of the LVAD emerging from the skin.
Thrombosis is the most common cause of pump failure.
Other issues you may encounter include dysrhythmias and battery failure. If there is a low battery, replace with a new one, but don’t replace both at once as disconnecting both batteries shut off the pump.
Order of checking a critically ill LVAD patient
1. Check the device and all its connections, address any alarms
No device sounds and patient unresponsive? CPR/ACLS. Do not restart LVAD if >30 minutes off do to risk of thrombi).
If unable to restart and patient still unresponsive, discuss ECMO
At the same time, continue to troubleshoot device attachments. Do not restart LVAD if >30 minutes off do to risk of thrombi). If unable to restart and patient still unresponsive, discuss ECMO
2. Listen for device sounds in the chest
If MAP < 60 think pump failure (thrombosis, arrhythmia, bleeding, dehydration)
3. Measure a manual BP using Doppler/US
Cardiac arrest
If at any time the patient is unresponsive, there is no measurable BP, and no indication the pump is functioning, CPR should be immediately initiated. Previously, CPR was discouraged out of fear of line dislodgement, but now newer guidelines advocate for CPR. One retrospective case series in 8 LVAD CPR cases showed no lines were dislodged. Defibrillate as indicated. Place the pads away from the LVAD unit. As CPR is occurring, the LVAD coordinator should be actively discussing with the surgery team about ECMO.
Assessment of an LVAD patient
Call the LVAD coordinator. No joke. When that patient hits the door of your ED, call the coordinator. They will absolutely want to be involved in the patient’s care and will often come to the ED as well. Doesn’t matter what the complaint is- call the coordinator.
Immediately inspect the LVAD. Look at all device connections, check the batteries. Connect to wall outlet if possible.
Auscultate over the heart for a “hum” noise, which indicates pump function. Any absence of this hum means pump failure.
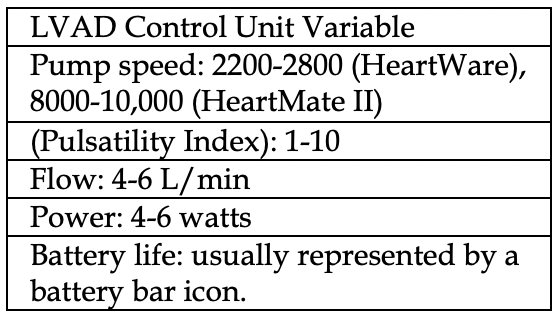
Look at the control unit and take note of critical readings (table, left). Attempt to correlate to potential causes (table below).
An EKG should be performed on patient arrival. It is reliable and along with telemetry can help determine rate & rhythm.
The physical exam isn’t full proof but needs to be done. Look for signs of poor perfusion or worsening heart failure, like skin temperature, capillary refill, JVD, or hepatomegaly.
Approach to the hypotensive LVAD patient (MAP <60): There will be a “low flow alarm” in these patients. Your primary concern is to rule out a suction event, hypovolemia, dysrhythmias, or RV failure. Place an arterial line sooner than later for accurate, continuous MAP monitoring. If able, ask the patient regarding GI bleeding and possible infectious sources.
Suction event: again, the most common cause of hypotension is a “suction event”. Remember that LVADs are highly preload dependent. Any drop in volume status (dehydration, infection, bleeding, vomiting/diarrhea) results in the inflow cannula attempting to pull blood from the LV, but it sucks down on the walls of the LV, therefore being partially occluded. Bedside echo might show excessive LV decompression with a small LV size. If suction event is suspected, give a fluid bolus first. The LVAD coordinator might reduce the LVAD speed and reassess the LV on echo again. Of note, LV suction can also be a manifestation of severe RV dysfunction or severe hypovolemia, or any other cause of decreased preload to the LV.
Bedside echo will demonstrate a small LV if there is a suction event occurring. The bedside echo will also show signs of RV strain suggestive of PE, or pericardial effusion suggestive of tamponade. If tamponade is present, do not attempt to perform a bedside pericardiocentesis as the patient likely has an intrapericardial hemorrhage and needs a surgical window. Of course, if the patient is in extremis, pericardiocentesis with ultrasound guidance may be attempted by an experienced EM physician, but this is beyond the scope of the review and this is unchartered territory in terms of guidelines.
Dysrhythmias: sustained, wide complex rhythms are highest in the first month of LVAD placement.
Thankfully, the treatment of these rhythms is the same as in non-LVAD patients if the patient is hemodynamically unstable.
Cardioversion is safe and pads should be placed away from the LVAD unit. The LVAD control unit should be briefly disconnected during the actual cardioversion event.
Bleeding: GI bleeding is the most common source. Acquired von Willebrand syndrome is another etiology of anemia as blood hurdles across the mechanical pump.
Any life-threatening bleeding will likely need reversal of anticoagulation, but this should be discussed actively with the patient’s LVAD team and the admitting ICU team. Reversing anticoagulation should never be the role of the EM physician until after discussing with the LVAD team.
Approach to the LVAD patient with a “high flow” alarm: look at the power level on the LVAD. If normal, this suggests distributive shock and sepsis. If the power level is high, its pump thrombosis until proven otherwise.
Pump thrombosis: The most common trigger of “high power” alarm. It occurs up to 2% of all LVAD patients annually.
A serum LDH will be elevated. Look at the urine as well- tea-colored urine suggests pump thrombosis.
Start heparin drip, admit to cardiac ICU. If the patient is hemodynamically unstable, thrombolytics should be used. Again, active discussions with the cardiology team should take place.
Infection: driveline infections are the most common location. The vast majority of infections (80%) occur <6 months after LVAD placement.
The presentations vary, ranging from sepsis to mild fever and warmth at the driveline.
If severe enough, or the patients delay care, the driveline infection can extend into the pump pocket and seed the endocardium.
Broad spectrum antibiotics are indicated, along with eventual LVAD replacement.
References
- Peberdy M, Guck J, Ornato J, et al. Cardiopulmonary resuscitation in adults and children with mechanical circulatory support: A scientific statement from the American Heart Association. Circulation 2017; 135:e1115.
- Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013; 32:157.
- Lanier GM, Orlanes K, Hayashi Y, et al. Validity and reliability of a novel slow cuff-deflation system for noninvasive blood pressure monitoring in patients with continuous-flow left ventricular assist device. Circ Heart Fail 2013; 6:1005.
- Bennett MK, Roberts CA, Dordunoo D, et al. Ideal methodology to assess systemic blood pressure in patients with continuous-flow left ventricular assist devices. J Heart Lung Transplant 2010; 29:593.
- Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant 2017; 36:1080.