Want to experience the greatest in board studying? Check out our interactive question bank podcast- the FIRST of its kind here: emrapidbombs.supercast.com
Author: Blake Briggs, MD
Peer Reviewer: Mary Claire O’Brien, MD
Introduction
Opioid abuse is an epidemic that has its roots in poor prescribing practices by physicians. In the US alone, ~5 million people were estimated in 2015 to have used heroin at least once in their lives. 329,000 reported use within the past month. 3,800,000 people report misuse of an opioid prescription in 2015. Nearly 70% of people who use heroin have been reported to also use prescription opioids. Illicit use of fentanyl is also on the rise, as this highly potent synthetic is used to “cut” heroin.
Signs of opioid overdose
-Depressed mental status
-Decreased respiratory rate
-Decreased tidal volume
-Decreased bowel sounds
Accelerate your learning with our EM Question Bank Podcast
- Rapid learning
- Interactive questions and answers
- new episodes every week
- Become a valuable supporter
-Miotic (constricted) pupils
A normal pupillary exam does not exclude opioid withdrawal (#louderforthoseintheback). For example, meperidine does not change pupils, and often the presence of adulterants or co-ingestants like sympathomimetics and anticholinergics can make the pupils appear normal or even enlarged.
The best predictor of opioid toxicity is respiratory rate <12.
Heart rate is typically normal or low normal (60-70).
Look for evidence of trauma, certainly check a fingerstick glucose. An EKG is never a bad idea, especially in cases of suspected self-harm or where loss of consciousness occurred.
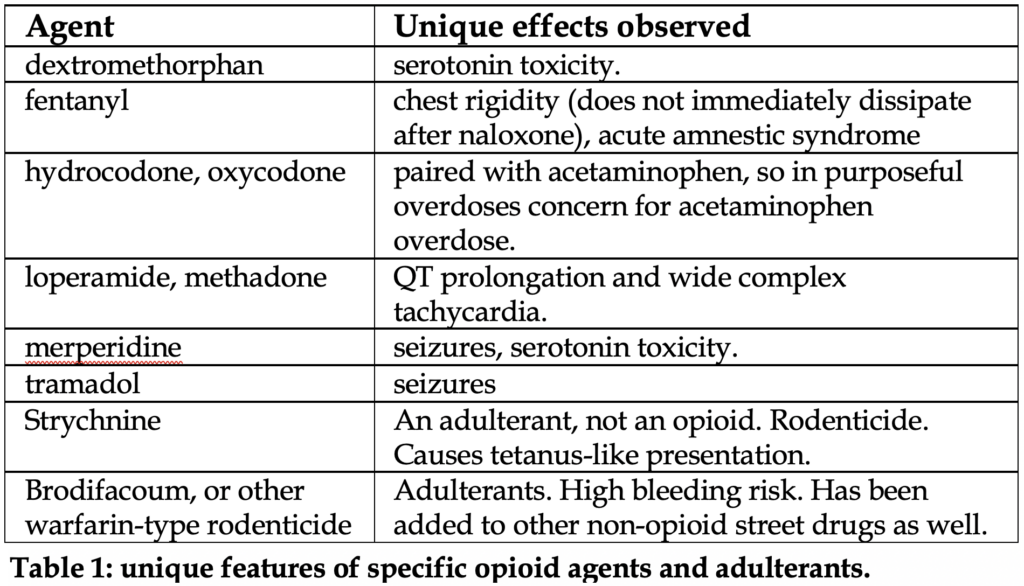
We do not recommend routinely checking urine toxicologic screens. In fact, in a young patient where opioid abuse is clinically suspected and responds well the naloxone, no evidence supports further diagnostic workup. Opioid overdose is a clinical diagnosis, and the status of drug screens does not change management. See Table 1 below for details on unique features of specific agents.
Differential diagnosis: in patients who present obtunded you must consider other sedative-hypnotics like clonidine, ethanol, benzodiazepines, and barbiturates. Hypoglycemia is a commonly missed medical diagnosis.
Clonidine, which has opioid-like effects, causes miosis but produces much more bradycardia and hypotension.
Management
As always, ABC’s come first. Pulse oximetry is useful for oxygenation but end tidal capnography is better. It can monitor ventilation which is more important in suspected opioid overdose patients. Hypoventilation is one of the earliest signs of respiratory decline, and EtCO2 >50 mmHg can predict complications of hypoventilation. Patients with respiratory depression or apnea require oxygen assistance, the latter with bag-valve mask while naloxone is being administered. Oxygenation reduces the risk of ARDS.
Naloxone: pure opioid antagonist, immediately displaces opioids at receptors. It reverses all respiratory and central nervous system depression within 1 minute. Reversal effects depend on the type and duration of the opioid(s) taken, from 20 minutes to an hour.
Unfortunately, many fail to recognize that naloxone triggers immediate opioid withdrawal, so “go big or go home” is the wrong answer in certain situations.
It really comes down to the patient so let’s keep it simple:
Is the patient in respiratory or cardiac arrest or in critical condition (key words: non-arousable or no spontaneous ventilations)?
Yes. By all means give large doses of naloxone.
If not, then give smaller doses of naloxone to avoid precipitating withdrawal.
That’s naloxone in a nutshell…. Kidding. Let’s hit the details.
Naloxone in life-threatening situations
If available, intranasal naloxone is recommended in life-threatening situations. Otherwise, initial dose: 2 mg IV rapid push. You can also do intramuscular or intraosseous for similar effects, but not as predictable as IV.
If the initial dose is partially effective, give the same dose again.
If no effects observed, give a higher dose.
There’s no literature on how many times you should administer naloxone and watch for an effect (and there probably will never be!), but in our experience we wouldn’t advise more than 10 mg total of naloxone if no clinical improvement is seen.
Naloxone in non-life-threatening situations
These are the patients who are “drowsy”. They are bradypneic, nodding off to sleep when you ask questions, and might have an oxygen saturation in the low 90’s.
We do not suggest slamming 0.4 mg IV naloxone. That will easily reverse an opioid in patients like this, but there are implications of putting patients into immediate withdrawal.
1. Staff safety: patients given full dose naloxone will wake up swinging fists, angry, and in pain. Keeping you and your staff safe is of the utmost importance.
2. Patient safety. Patients who entire full withdrawal are more likely to leave AMA and proceed to abuse opioids again… often very, very soon. They are at risk of using higher opioid doses as well because they are in active withdrawal.
We advise mixing 0.4 mg/mL with 9 mL normal saline in a syringe (you now have 40 mcg/mL). Titrate to effect via IV by administering very slowly. Thanks to pharmacyjoe.com for this simple approach.
The patient will open their eyes and become more alert within 1 minute. If not, continue to give the solution and add more as needed.
When an opioid effect is expected to be prolonged (e.g. massive overdose, suicide attempt, methadone usage), a continuous infusion or naloxone should be used. You likely have this stocked at your hospital, but it’s easy to make: mix 4 mg naloxone in 100 mL D5W.
The initial naloxone infusion rate should be 2/3 of the naloxone dose that reversed the patient’s symptoms.
Example: initial bolus dose which reversed symptoms = 2 mg; Start infusion at 1.3 mg/hr.
Titrate the infusion as needed: increase by 0.1 to 0.2 mg/hr if symptoms return.
To wean off the infusion:
1. Decrease by 0.1 to 0.2 mg/hr every 2 hours.
2. Check the patient for signs/symptoms.
3. If decreased respiratory rate or responsiveness is noted, return to the previous rate and attempt to decrease again in 1 to 2 hours.
Monitoring post-naloxone
Here comes the hard part. Its controversial how long patients who received naloxone should be watched in the ED. The underlying concern is that the duration of naloxone is shorter than the duration of most opioids. So, if naloxone wears off in about 40 minutes, close monitoring is needed for a few hours to be sure there are no recurrent symptoms. No one really agrees on the amount of time to observe, and that’s honestly never going to happen. Each patient is different, and each street substance is different. Even if a study is done in a certain region of the world, what’s to say a different illicit substance is being sold elsewhere which has different effects?
When deciding on observation and disposition, it’s not an easy decision and involves a lot of questions. Firstly, it matters what route they ingested the opioid. IV and IN are more predictable. Also, you must think about the patient’s underlying health, presence of other co-ingestants, especially other sedatives. Discharging a patient “as soon as safely possible” is not always the right answer.
When the choice is made to discharge, bystander-administered naloxone (available as IM or IN routes) should be prescribed. Providing the patient, family members, or friends with this medication can reduce overdose mortality. In fact, one study showed implementation of a comprehensive opioid overdose prevention program decreased deaths from 46 to 29 per 100,000.
Complications of opioid toxicity
One unique complication is lung injury and noncardiogenic pulmonary edema; it is rare (<3% of patients receiving naloxone).
The pathophysiology is unclear, but it can be a major adverse effect of opioid overdose, especially in morphine, heroin, and methadone. It seems that the trigger is iatrogenic from reversal of opioid toxicity with naloxone. Rapid precipitation of withdrawal in the setting of elevated pCO2 causes a surge of catecholamines, therefore increasing afterload and vascular permeability in the lungs. This is another reason to use small doses of naloxone if possible.
It has a rapid onset, often with crackles, hypoxia, and frothy sputum. Expect hypertension and tachcardia.
There are no published, evidence-based guidelines on how to manage this. It will not be on board exams. Supportive management is indicated: supplemental oxygen, likely with NIPPV, is standard of care. Diuretics have been quoted as helpful, along with nitroglycerin if patients are hypertensive.
References
1. Pharmacy Joe. “Episode 25: how to dose naloxone for opioid reversal.” https://www.pharmacyjoe.com/how-to-dose-naloxone-for-opioid-reversal/. Published 10/15/2015. Date accessed: 8/10/20.
2. Jiwa N, Sheth H, Silverman R. Naloxone-Induced Non-Cardiogenic Pulmonary Edema: A Case Report. Drug Saf Case Rep. 2018;5(1):20. Published 2018 May 10. doi:10.1007/s40800-018-0088-x
3. Cole, Jon. “Tox and Hound – Great! Naloxone worked! Now what?” Published 11/4/2019. Date accessed: 8/7/2020. https://emcrit.org/toxhound/naloxone-now-what/
4. 2015 National Survey on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Administration, Rockville, MD 2016.
5. Rosenblum A, Parrino M, Schnoll SH, et al. Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug Alcohol Depend 2007; 90:64.
6. Darke S, Zador D. Fatal heroin ‘overdose’: a review. Addiction 1996; 91:1765.
7. Binswanger IA, Blatchford PJ, Mueller SR, Stern MF. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med 2013; 159:592.
8. Leach D, Oliver P. Drug-related death following release from prison: a brief review of the literature with recommendations for practice. Curr Drug Abuse Rev 2011; 4:292.
9. Ghoneim MM, Dhanaraj J, Choi WW. Comparison of four opioid analgesics as supplements to nitrous oxide anesthesia. Anesth Analg 1984; 63:405.
10. Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Med 1991; 20:246.
11. Viglino D, Bourez D, Collomb-Muret R, et al. Noninvasive End Tidal CO2 Is Unhelpful in the Prediction of Complications in Deliberate Drug Poisoning. Ann Emerg Med 2016; 68:62.
12. Barash JA, Ganetsky M, Boyle KL, et al. Acute Amnestic Syndrome Associated with Fentanyl Overdose. N Engl J Med 2018; 378:1157.
13. Eggleston W, Nacca N, Marraffa JM. Loperamide toxicokinetics: serum concentrations in the overdose setting. Clin Toxicol (Phila) 2015; 53:495.
14. Ashbourne JF, Olson KR, Khayam-Bashi H. Value of rapid screening for acetaminophen in all patients with intentional drug overdose. Ann Emerg Med 1989; 18:1035.
15. Mills CA, Flacke JW, Flacke WE, et al. Narcotic reversal in hypercapnic dogs: comparison of naloxone and nalbuphine. Can J Anaesth 1990; 37:238.
16. Berlot G, Gullo A, Romano E, Rinaldi A. Naloxone in cardiorespiratory arrest. Anaesthesia 1985; 40:819.
17. Goldfrank L, Weisman RS, Errick JK, Lo MW. A dosing nomogram for continuous infusion intravenous naloxone. Ann Emerg Med 1986; 15:566.



